6 MFDS codes for Products and Ingredients in Post-marketed Domestic Cases
MFDS has published a Regional Drug Dictionary with Product code and Ingredient code assigned for each Product and the set of Ingredients in that Product.
MFDS Product Code – 9-digit unique code per Product
MFDS Ingredient Code – 7-digit unique code per Ingredient
MFDS uploads the Drug Product License details spreadsheet in nedrug.mfds.go.kr, and this is refreshed in real time (on a daily basis). The location for file download is:
For post-marketed domestic cases, it is mandatory to transmit the MFDS Product code or MFDS Ingredient code for every Company and Non-company Product in the KR specific regional data elements (outlined in the table below) in E2B(R3) report as per the Business rules.
To achieve this, four new Case Form fields are introduced in Oracle Argus Safety.
It is required that companies manually enter the MFDS assigned Product code and Ingredient code in the Case Form fields as outlined in the table below.
| Element ID | Element Description | Case Form Field |
|---|---|---|
| D.8.r.1.KR.1b | Medicinal Product ID
(Patient Past drug Therapy) |
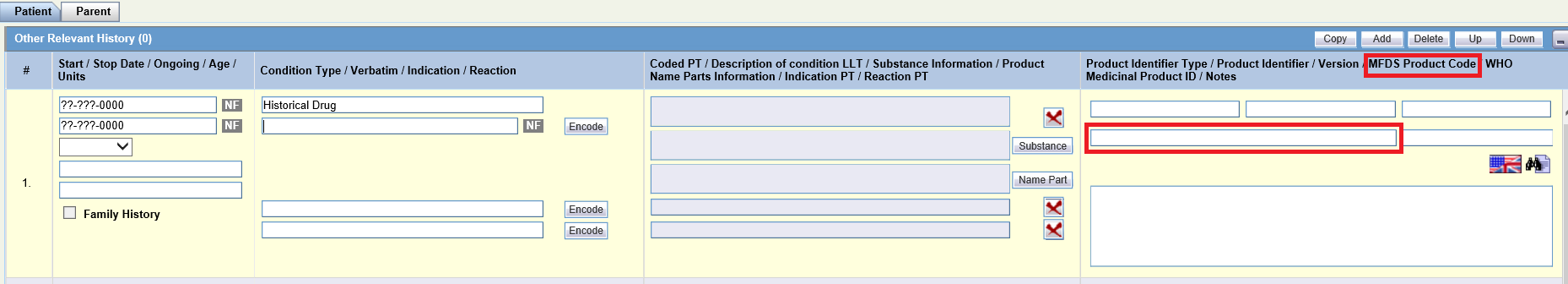
Patient tab > Other Relevant History > MFDS Product Code |
| D.10.8.r.1.KR.1b | Medicinal Product ID
(Parent Past drug Therapy) |
Parent tab > Other Relevant History > MFDS Product Code |
| G.k.2.1.KR.1b | Medicinal Product ID
(Suspect/Concomitant/Interacting Products) |
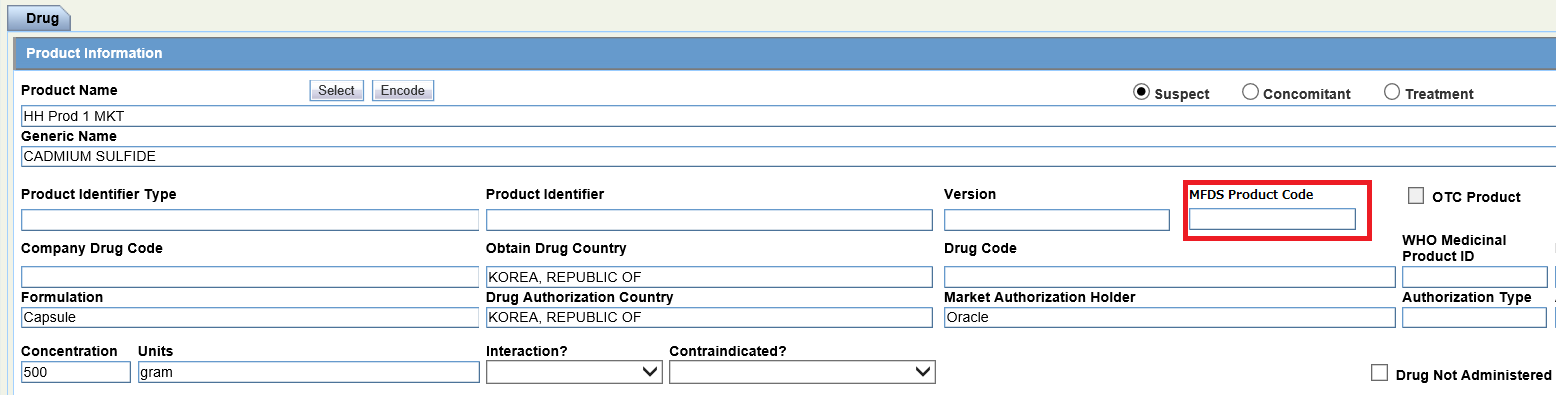
Product tab > Product Information > MFDS Product Code |
| G.k.2.3.r.1.KR.1b | Substance ID
(Ingredients of Suspect/Concomitant/Interacting Products) |
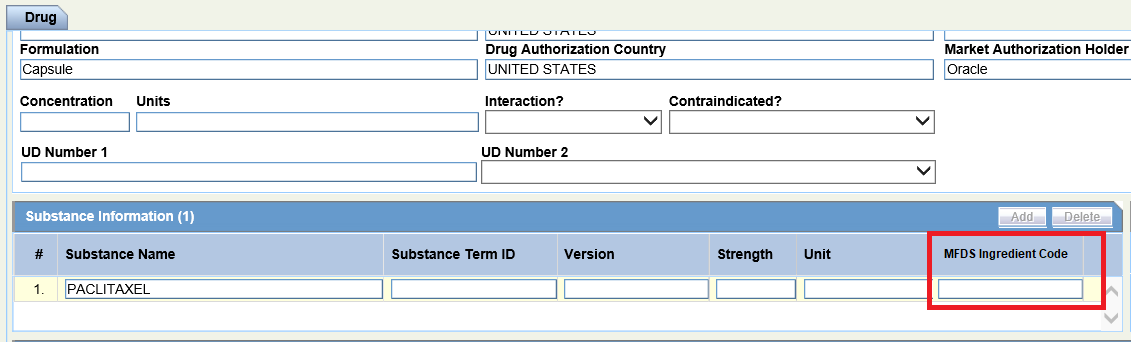
Product tab > Substance Information > MFDS Ingredient Code |
- Patient/Parent tab > Other Relevant History > MFDS Product Code:
- Product tab > Product Information > MFDS Product Code
- Product tab > Substance Information > MFDS Ingredient Code
Note:
It is in Oracle Argus Safety roadmap to enhance data entry and to provide a way to automatically populate MFDS Product Code and MFDS Ingredient Code when any Product is added to case.- For company products, this requires association of MFDS-specific codes in Console > Product > License Configuration.
- For non-company products, this requires association of MFDS-specific codes in WHO drug dictionary tables. Based on the discussions with WHO-UMC, it is understood that WHO-UMC is working with MFDS to provide this mapping between WHO drug/ingredient codes and MFDS specific codes.
- The official release date of the WHODrug Link Korea first version is March 1st, 2021. This release is compatible with the March 2021 WHODrug Global release.