1 Study configuration basics
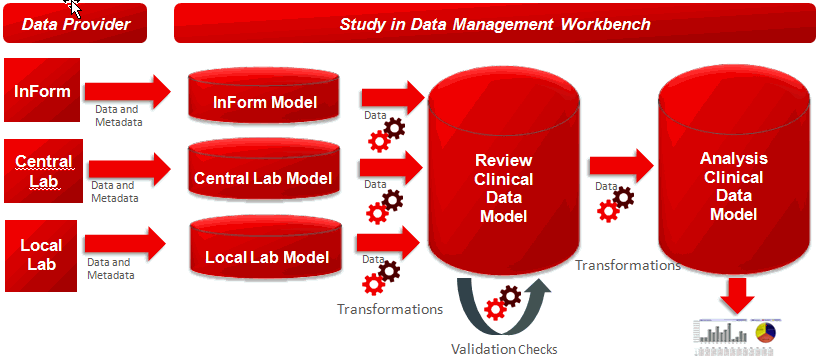
The following figure illustrates how you can provide data from an electronic clinical data system (such as InForm), labs, or file systems to transform and validate the data in a study using Oracle Health Sciences Data Management Workbench.
For details on DMW concepts, see this video:
Table 1-1 High-level steps for creating a study
| Task | More information |
|---|---|
|
Begin study creation. |
Specify a name and other attributes. See Create a study. |
|
(Optional) Apply a study template. |
A study template includes clinical data models, transformations, validation checks, and custom listings. You can modify or delete any of these as needed. See Use a study template for details. |
|
Set up importing data from InForm. |
Create an input clinical data model of type InForm and set up the InForm Connector for the study. The Connector creates table metadata exactly as it exists in the InForm database. See Create a clinical data model for InForm data. |
|
Set up importing data from labs and other sources using files. |
The system administrator must set up File Watcher for the system and for your study. Then create an input clinical data model of type File. See Create a file input clinical data model. |
|
Create or copy target clinical data models. |
A target clinical data model is a collection of tables used together for a purpose such as reviewing or analyzing data. You can:
|
|
Create or copy transformations. |
A transformation reads data from one or more tables in one or more clinical data models and writes data to a table in a different model. See Set up data transformations. |
|
Create or copy validation checks. |
Validation checks, or edit checks, check data for a condition and create discrepancies on faulty data. See Create validation checks. |
|
Create public custom listings and filters. Set up static reference data. |
See Create custom programs, listings, and filters, and set up reference data. |
|
Integrate with Thesaurus Management System (TMS) for coding (optional). |
|
|
Load data and run transformations and validation checks from the Study Manager page. |
See: |
|
Upgrade models, transformations, and validation checks to Quality Control (optional) and then Production. |
See also: