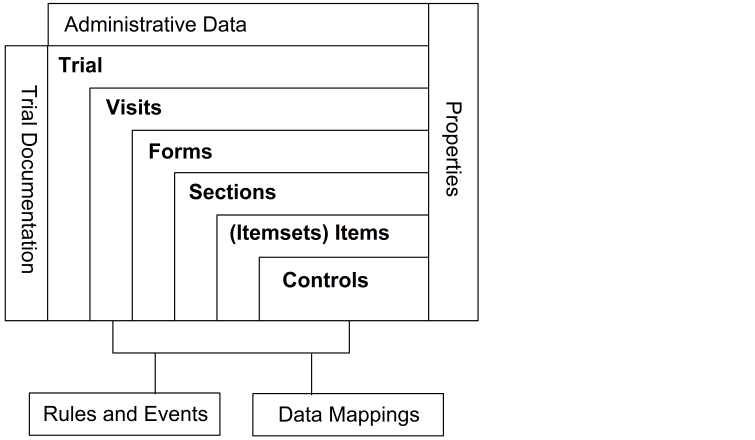
Trial building blocks
The components of a trial have a hierarchical relationship with the trial at the top level and the controls on individual forms at the bottom. The building blocks that make up the lower-level components are reusable in higher-level components. Thus, the same form can appear in multiple visits, the same item can appear in multiple forms, and the same controls can appear in multiple items.
The major building blocks in a trial are as follows:
- A trial consists of a set of visits.
- Each visit consists of a set of forms, most commonly CRFs.
- Forms consist of sections.
- Each section has an itemset or one or more items. An itemset is made up of multiple items.
- Each item has one or more controls.
Each of these components is defined with a set of properties, which specify the characteristics and behavior of the component.
In addition to the building-block components of a trial, a trial definition is associated with the following related components:
- Administrative data specifies the users, sites, groups, and rights in a trial.
- Trial documentation provides online information about a trial protocol and details about CRF items.
- Rules and events, which work in the context of one or more specific items, enable validation or calculation of data items and automatic generation of queries.
- Data mappings specify mappings between the items in a trial database and any of the following destinations:
- Customer-Defined Database (CDD).
- Clintrial database.
- Import file formatted for upload to the Oracle Clinical tool.
- Calculated control designed as the destination of a value supplied by an autocode utility.
The following figure illustrates the building-block components and other components of a trial definition.