About trials
When an investigator collects data from a patient during a patient visit, the data are transferred to a set of forms. Collectively the forms for a particular patient visit to a site make up a visit. The collection of all trial visits is called a trial. The definition of a trial contains definitions of the trial’s visits, which in turn contain the definitions of forms, in a hierarchical structure.
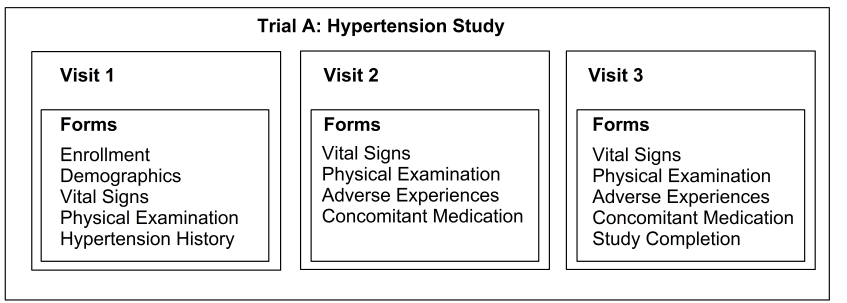
A trial definition represents all of a trial’s forms and visits at a particular point in time. If a form changes, or a form or visit is added or dropped, a new trial definition is required.
You can map each trial to one or more sites and to a set of trial documentation. Each time a component of a trial changes, you create a new trial and map the new version to the sites and trial documentation to which it applies. With this mapping, you can ensure that when a protocol change requires a change in forms, the new forms come into effect as each site’s IRB approves the protocol change.



