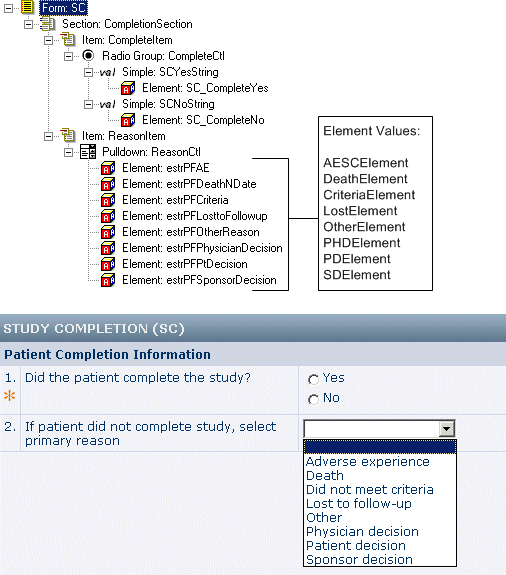
Separate study completion and noncompletion reason items
In this example:
- Study completion is captured in an item that uses a radio control with the PF_SC_COMPLETECTL UUID. The Yes and No choices are defined by element components with the PF_SC_STUDY_COMPLETE and PF_SC_STUDY_INCOMPLETE UUIDs.
- Incomplete reason is captured in an item that uses a pulldown list defined with the PF_SC_REASONCTL UUID. Each selection in the pulldown list is associated with an element component defined with a value.
- Each element component has a corresponding text resource in which the UUID includes the element’s value.
The following MedML file defines report column headings for noncompletion reasons:
<?xml version="1.0"?>
<MEDMLDATA xmlns="PhaseForward-MedML-Inform4">
<RESOURCE
UUID="PF_SC_REASONCTL_AESCElement" DESCRIPTION="SC AE column heading"
DATATYPE="TEXT" TEXT="Adverse Experience"/>
<RESOURCE
UUID="PF_SC_REASONCTL_DeathElement" DESCRIPTION="SC Death column
heading" DATATYPE="TEXT" TEXT="Death"/>
<RESOURCE
UUID="PF_SC_REASONCTL_CriteriaElement" DESCRIPTION="SC Criteria
column heading" DATATYPE="TEXT" TEXT="Did Not Meet Criteria"/>
<RESOURCE
UUID="PF_SC_REASONCTL_LostElement" DESCRIPTION="SC Lost column
heading" DATATYPE="TEXT" TEXT="Lost to Follow-Up"/>
<RESOURCE
UUID="PF_SC_REASONCTL_OtherElement" DESCRIPTION="SC Other column
heading" DATATYPE="TEXT" TEXT="Other"/>
<RESOURCE
UUID="PF_SC_REASONCTL_PHDElement" DESCRIPTION="SC PHD column heading"
DATATYPE="TEXT" TEXT="Physician Decision"/>
<RESOURCE
UUID="PF_SC_REASONCTL_PDElement" DESCRIPTION="SC PD column heading"
DATATYPE="TEXT" TEXT="Patient Decision"/>
<RESOURCE
UUID="PF_SC_REASONCTL_SDElement" DESCRIPTION="SC SD column heading"
DATATYPE="TEXT" TEXT="Sponsor Decision"/>
</MEDMLDATA>



