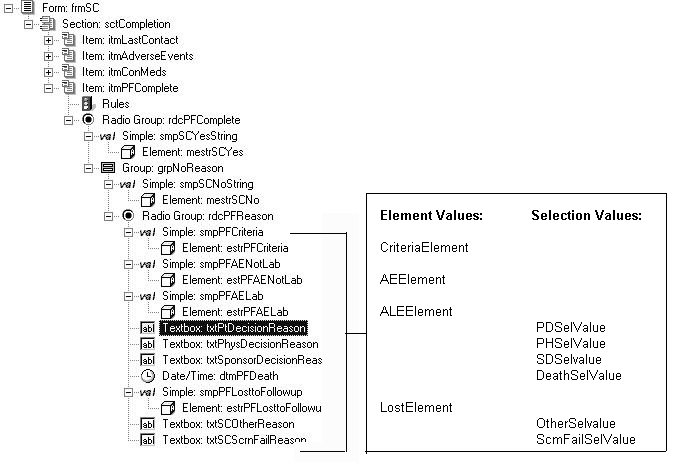
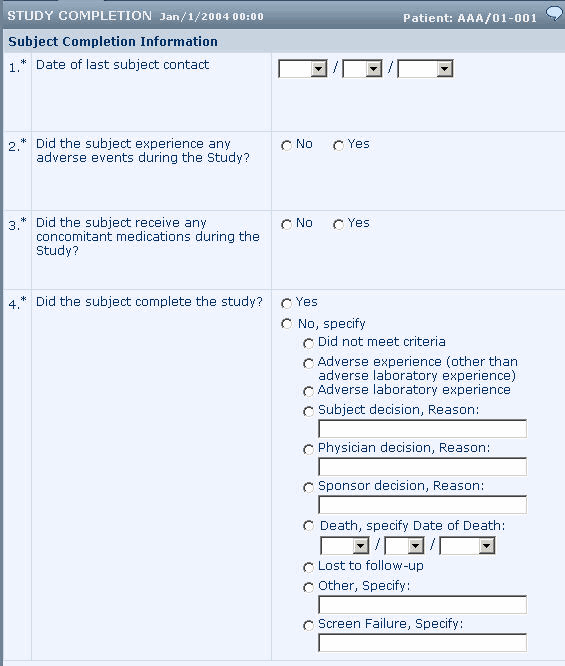
Same item for study completion and noncompletion reason
In this example:
- Study completion and noncompletion reason are captured in a single item that uses a radio control with the PF_SC_COMPLETECTL UUID.
- The Yes choice is defined by an element component with the PF_SC_STUDY_COMPLETE UUID. The No choice is defined by a radio control with the PF_SC_REASONCTL UUID. The choices in the radio control are defined by simple or group controls. By using group controls you can capture additional information along with each noncompletion reason.
Note: In this example the form does not use an element component with the PF_SC_STUDY_INCOMPLETE UUID. If you use the InForm application to save the trial by component, the application does not save the PF_SC_STUDY_INCOMPLETE element component because it is not used in the trial. Therefore, in this case you must load that element component into the trial database separately with MedML and the MedML Installer tool. The VALUE attribute of the study element component with the PF_SC_STUDY_INCOMPLETE UUID that you load with the MedML Installer tool must be the same as the Selection Value property of the radio control with the PF_SC_REASONCTL UUID.
- The UUID for each text resource that is associated with a noncompletion reason control reflects the element component Value property for each reason defined with a simple control or the Selection Value property for each reason defined with any other type of control.
The following MedML file defines report column headings for noncompletion reasons:
<?xml version="1.0"?>
<MEDMLDATA xmlns="PhaseForward-MedML-Inform4">
<RESOURCE UUID="PF_SC_REASONCTL_CriteriaElement"
DESCRIPTION="SC Criteria column heading" DATATYPE="TEXT" TEXT="Did Not Meet Criteria"/>
<RESOURCE UUID="PF_SC_REASONCTL_AEElement"
DESCRIPTION="SC AENonLab column heading" DATATYPE="TEXT" TEXT="Adverse Experience Non-Laboratory"/>
<RESOURCE UUID="PF_SC_REASONCTL_ALEElement"
DESCRIPTION="SC AELab column heading" DATATYPE="TEXT" TEXT="Adverse LaboratoryExperience"/>
<RESOURCE UUID="PF_SC_REASONCTL_PDSelValue"
DESCRIPTION="SC PTD column heading" DATATYPE="TEXT" TEXT="Patient Decision"/>
<RESOURCE UUID="PF_SC_REASONCTL_PHDSelValue"
DESCRIPTION="SC PHD column heading" DATATYPE="TEXT" TEXT="Physician Decision"/>
<RESOURCE UUID="PF_SC_REASONCTL_SDSelValue"
DESCRIPTION="SC SD column heading" DATATYPE="TEXT" TEXT="Sponsor Decision"/>
<RESOURCE UUID="PF_SC_REASONCTL_DeathSelValue"
DESCRIPTION="SC Death column heading" DATATYPE="TEXT" TEXT="Death"/>
<RESOURCE UUID="PF_SC_REASONCTL_LostElement"
DESCRIPTION="SC Lost column heading" DATATYPE="TEXT" TEXT="Lost to Follow-Up"/>
<RESOURCE UUID="PF_SC_REASONCTL_OtherSelValue"
DESCRIPTION="SC Other column heading" DATATYPE="TEXT" TEXT="Other"/>
<RESOURCE UUID="PF_SC_REASONCTL_ScrnFailSelValue"
DESCRIPTION="SC Scrn Fail column heading" DATATYPE="TEXT" TEXT="Screen Failure"/>
</MEDMLDATA>



