PMDA Information: Comments Tab
The following figure depicts the Comments sub-tab of the PMDA Information tab:
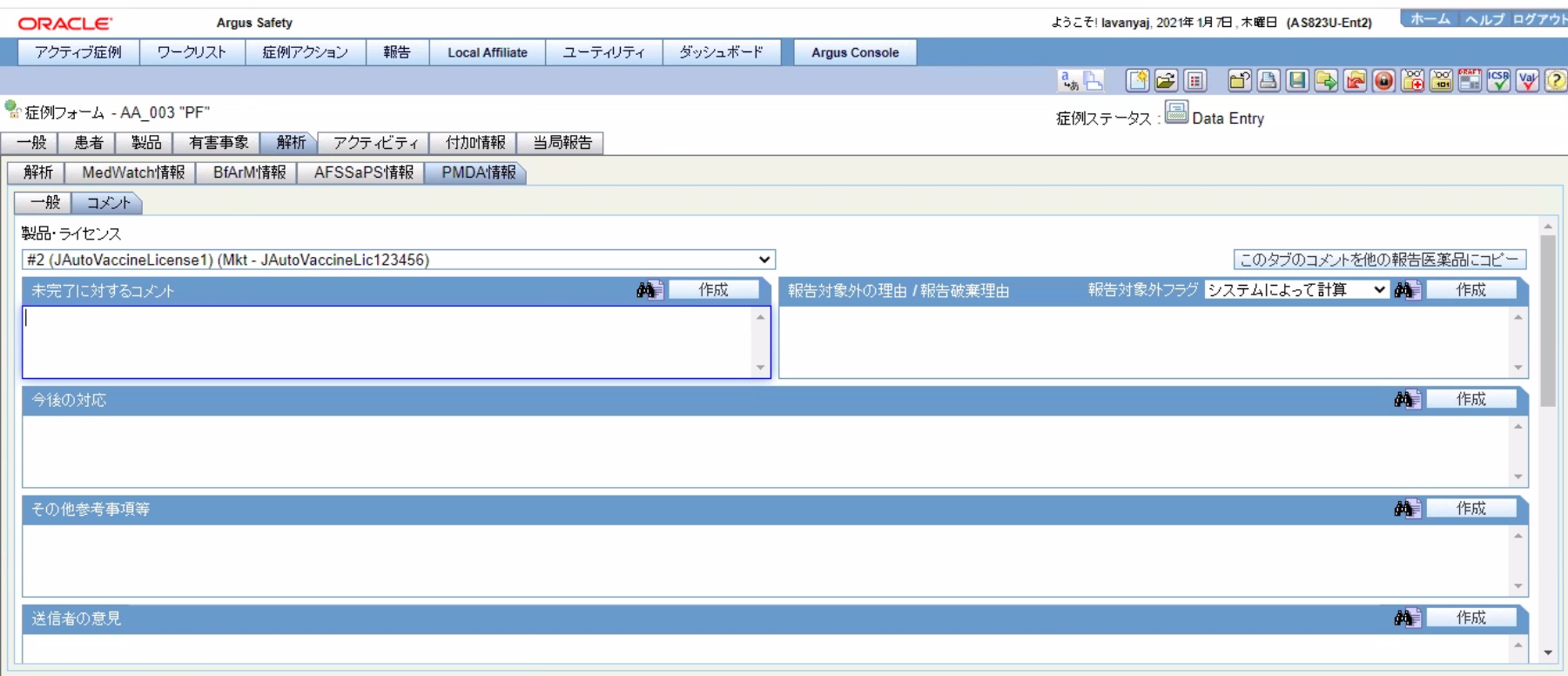
The following table lists the fields in the Comments tab:
Table 1-5 Fields in the Comments tab
| # | Field Name | Description |
|---|---|---|
|
1 |
General |
This represents the Sub-tab of the PMDA Information tab. |
|
2 |
Comment |
This represents the Sub-tab of the PMDA Information tab. |
|
3 |
Product, License No |
This is a drop-down field. It allows you to enter J specific narrative information based on the each suspected drug license. |
|
4 |
Comment on incomplete |
This field is unique to Japan. This field is an editable text that maps to DTD element J.7 as per PMDA. In case of an incomplete report, input a comment in J.7 <mhlwadmicsrcommentsincomplete> for the report being incomplete. |
|
5 |
E.g. Cure_All MKT (xxxxxxxxxxxxxxx) |
This field represents the E.g. Sample License for which the narratives are being written. All the narratives written in the various text boxes are applicable to this License. |
|
6 |
Counter measure for the future |
This field is unique to Japan. This field is an editable text that maps to the J.9<mhlwadmicsrcountermeasures> DTD element as per PMDA. The company inputs the counter measures for the future based on the evaluation made by the reporting company on the concerned adverse effect infection, etc. For foreign case, the company inputs the counter measures taken by the Japanese reporting company, and not the foreign company. The field size is 10000J. |
|
7 |
Other references |
This field is unique to Japan. This field is an editable text that maps to the J.10/J2.11<mhlwadmicsrreporttimesevent.> DTD element as per PMDA for E2B (R2) or E2B (R3) respectively. |
|
8 |
Comment of sender |
This field is unique to Japan. This field is an editable text. This refers to the B.5.4 < sendercomment > element. |
|
9 |
Remarks 1 |
This field is unique to Japan. This field is an editable text that maps to the J.13.1 DTD element as per PMDA. The DTD element name is:<mhlwadmicsrremarks1> |
|
10 |
Remarks 2 |
This field is unique to Japan. This field is an editable text that maps to the J.13.2 DTD element as per PMDA. The DTD element name is:<mhlwadmicsrremarks2> |
|
11 |
Remarks 3 |
This field is unique to Japan. This field is an editable text that maps to the J.13.3 DTD element as per PMDA. The DTD element name is:<mhlwadmicsrremarks3> |
|
12 |
Remarks 4 |
This field is unique to Japan. This field is an editable text that maps to the J.13.4 DTD element as per PMDA. The DTD element name is:<mhlwadmicsrremarks4> |
|
13 |
Copy the comments in this tab to other reporting licenses |
This button copies all the comments in the UI to other licenses in the list. When there is only one product, the button is disabled. |
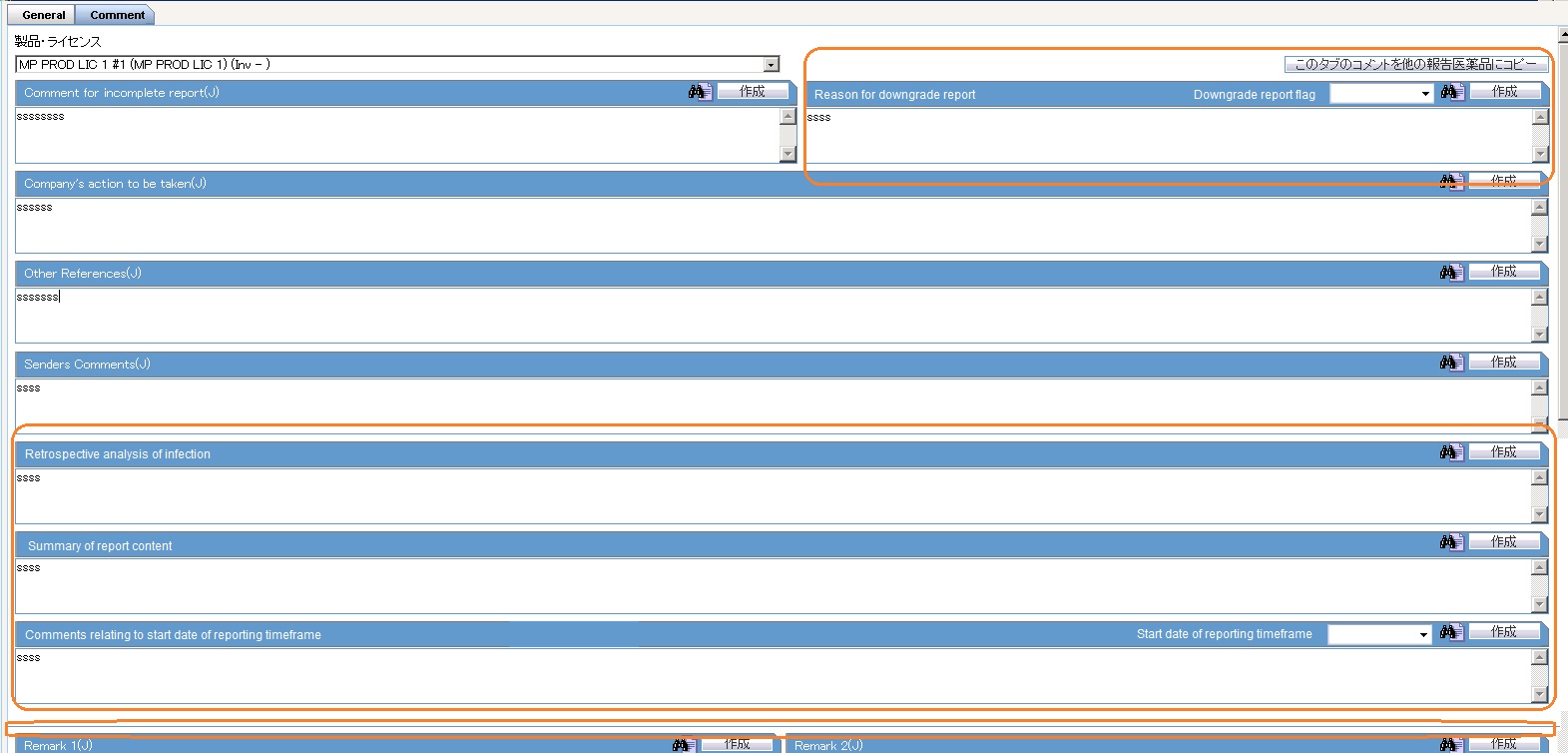
The following new fields have been added to enter information for each license that is available in the Product, License No drop-down:
- Retrospective analysis of infection
- Summary of report content
- Comments relating to start date of reporting timeframe
- Start date of reporting timeframe drop-down list
- Reason for downgrade report
- Downgrade report flag drop-down list with the options System Calculated, Downgrade, Nullification
Functionality Changes
The following are the functionality changes for the Comments tab:
-
This tab captures narratives data for various J DTD elements that are reported in E2B reports being submitted to PMDA.
For details, refer to the DTD Mapping document.
-
Product, License drop-down contains the same suspected drug licenses listed in the PMDA General tab. This is to include distinct narratives for each license. Each comment is applied to the selected License Number within the drop-down field. So a unique narrative field is available for each license. In the Product, License drop-down, licenses are displayed in the following format:
Trade Name (License number)
Depending on which license is picked, appropriate Narrative fields is displayed.
-
When Copy the comments in this tab to other reporting licenses is clicked, a message Comments already exist for other licenses and will be overwritten. Do you wish to continue? is displayed. If you click OK, the copy process is continued, and if you click Cancel, the copy is canceled.
-
This tab has auto text generation functions on each text box:
- UI: (buttons are located on the right side of each binocular icon).
- The label of the button Generate
- The function of the button is the same as Generate buttons in the Analysis tab. The PMDA tab does not have English or other languages equivalent fields, therefore, the auto narrative functions populate the texts by using Japanese Narrative template of the Console Configuration.
-
The following new comments fields are added in the Product License No dropdown list:
- Retrospective analysis of infection (J2.9): Captures the information from analysis of infection cases for blood products as specified in the PMDA Guideline for Retrospective Review of Blood Products. This will be used only for reporting of infection case reports.
- Summary of report content (J2.16): Capture the summary of the main issues found in the respective report for Study Reports and Reports on Overseas > Actions.
- Comments relating to start date of reporting timeframe (J2.2.2): Captures any user comments for explaining the value of J2.2.1 – Start date of Reporting Timeframe element in the PMDA E2B (R3) report.
Start date of reporting timeframe: This is the corresponding dropdown list added to capture the manual user input for J2.2.1 - Start date of Reporting Timeframe element in the PMDA E2B (R3) report. This dropdown list has the following value:
- System-Calculated
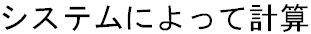 : This is the default value and the value for all the Aware date
(J) (Japanese date format) i.e. the date's corresponding to significant
follow-ups for that case. you may change the value manually.
: This is the default value and the value for all the Aware date
(J) (Japanese date format) i.e. the date's corresponding to significant
follow-ups for that case. you may change the value manually.
-
Reason for downgrade/Nullification report (J2.8.2/C.1.11.2): Captures the reason for downgrading/nullification of a report.
Downgrade report flag: A corresponding dropdown list is added to capture the manual user input to indicate to the application if next PMDA E2B/Paper report for the license is scheduled as a downgrade or nullification in the scenario when no reporting rule matches. This dropdown list has the following values:
- System-Calculated
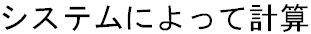 (Default)
(Default)
- Downgrade
- Nullification
-
To edit both Comments relating to start date of reporting timeframe and Reason for downgrade/Nullification report dropdowns after the case lock (globally and locally locked), set the profile switch Allow user to update the "Reason for Downgrade/Nullification report" and "Comments for start date of reporting timeframe" after the case is locked(globally and locally locked) as Yes.
Parent topic: PMDA Information tab