PMDA Information: General Tab
The following figure depicts the General sub-tab of the PMDA Information tab:
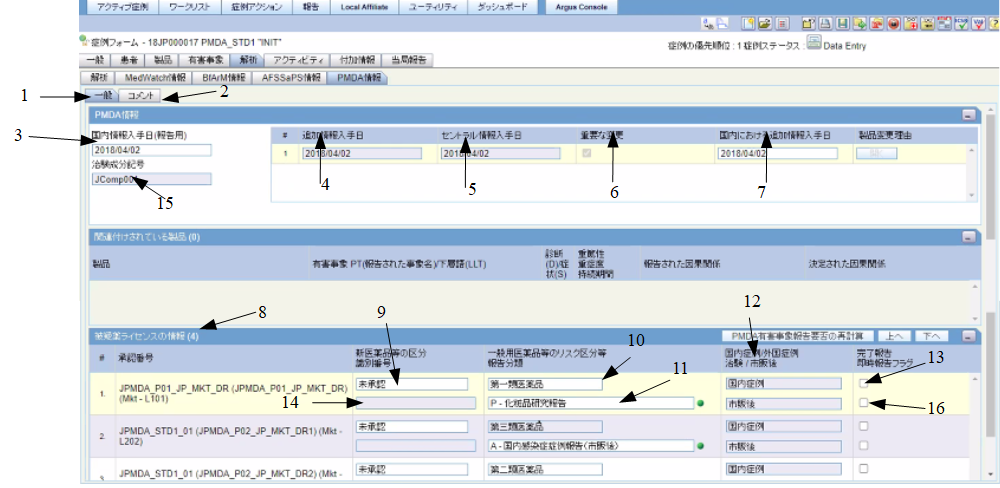
The following table lists the fields in the General tab:
Table 1-1 Fields in the General tab
| # | Field Name | Description |
|---|---|---|
|
1 |
General |
This represents the name of the sub-tab under PMDA tab. |
|
2 |
Comment |
This represents the name of the sub-tab under PMDA tab. |
|
3 |
Japan first information Received Date (For Reporting) |
This field represents the editable text box which displays the date the case was received by the Japanese pharma company (initial receipt date captured during case book-in). The format is YYYYMMDD. In case of a local case, this date represents the Case Initial Receipt date. In global case, this date is automatically populated by the date first opened by J user. The value is stored in the case when you perform direct/indirect Save. For example, accept from worklist stores the value indirectly. |
|
4 |
Follow-up received date |
This field represents the non-editable text box which displays the date the case follow-up information was received by the pharma company. If the follow-up is marked significant, the Japan Follow-up Received date is used as the reference date to schedule the reports. The format is YYYYMMDD. |
|
5 |
Safety received date / or Central received date |
This field represents the non-editable text box which displays the date the Safety group received the case. The format is YYYYMMDD. |
|
6 |
Significant Changes |
This is a non-editable check box to indicate whether the follow-up is significant or not. If the follow-up is significant, the Japan Follow-up date overrides the date used for report scheduling. |
|
7 |
Japan follow-up received date |
This field represents the editable text box which displays the date the case follow-up information is received by the pharma company. If the case is originated in some country other than Japan, this date represents the date first opened by the J user after the follow-up. If the follow-up is marked significant, the Japan follow-up received date is used as the reference date to schedule the reports. The format is YYYYMMDD.The time stamp of Case Open date done by first Japanese user after the latest foreign follow-up is automatically populated.The value is stored in the case when you perform direct/indirect Save. For example, accept from worklist stores the value indirectly. |
|
8 |
Drug name and license number |
The number of rows in this section is equal to the number of reports that need to be submitted to PMDA for this case. One report is scheduled for each of the Marketed License. One report each is scheduled for each Study License. The field is read only and show concatenation of Trade Name with License Number in brackets. |
|
9 |
New Drug Category |
This field represents the drop-down to select License Category LM to select value for E2B J.8 item. |
|
10 |
Risk category of OTC drugs |
This drop-down lists all the values from Argus Console > Business Configuration > License Configuration > Risk Category of OTC drugs. The value configured for that license in Argus Console > Business Configuration > License Configuration > Risk Category of OTC drugs is populated by default and you can change it manually. The drop-down is available only if the license for the corresponding record is configured by selecting the OTC Product check box on Argus Console > Business Configuration > License Configuration. |
|
11 |
Japan Reporting category |
This field represents a drop-down list containing Reporting Category LM. This is a mandatory field for Argus J user. The selection of this field determines the report being created (ADR, Infection, Research, or Measures in foreign countries). The entries shown in this list are determined at run time by the values selected in items 9,10 and 12.When Reporting Category is not selected, the license does not create the report. The format of the value displayed in this drop-down is: [Reporting Category] - [Description] |
|
12 |
Domestic Case / Foreign CaseClinical / Marketed |
There are two fields which are non-editable text boxes. The first shows whether the case is foreign (if the country of incidence is not equal to Japan) or domestic (if the country of incidence is Japan).The second field shows the license type: Investigational, if the license on which the ICSR is based on is for an investigational drug, device, or vaccine. Marketed, if the license on which the ICSR is based on is for a marketed drug. |
|
13 |
Completion Report (Case Complete) |
This check box indicates the completion of the case report. A new Case Complete check box field is added to the License List box. This field is the driver for the E2b field J.6. A separate J.6 must be recorded for each ICSR. By default, this box is not checked. |
|
14 |
PMDA Identification Number |
The PMDA Number is displayed in the PMDA tab where all the ICSR licenses are displayed. This is the PMDA Acknowledgment Number given in Ack message item B.1.3. This number is used in J.4b from the first follow-up report. |
|
15 |
Clinical Compound Number in the study of this case |
This field is read only when the study is selected from the Study look up. When the study information is manually entered, Clinical Compound Number is editable. If there is no study information, the field is disabled. |
|
16 |
Urgent Report |
Select this option to mark the report as urgent. |
Functionality Changes
The following are the functionality changes for the PMDA Information > General tab:
-
This tab is unique to Argus J module implemented system. It also displays the number of reports to be submitted for the case. Each row in the lower section represents a report. The number of reports to be submitted for a case is determined as per the following logic:
- General Rule
- Reports are submitted for Marketed or Investigational licenses in Japan only for the suspect company products in the case.
- All Japanese licenses (including hidden) listed in the event assessment are listed.
- All suspected products with license and all Investigational licenses are listed in the PMDA General Tab.
- Priority order of the Suspected product (Drug, Device, Vaccine) list is:
Primary Suspected Product License
Product entry order in the Case Form
Marketed license, then Investigational
- The license is displayed as the Trade Name and the License Number in parenthesis.
- General Rule
-
Japanese Receipt Date and Follow Up Dates table:
It captures the Japanese received date label as Japanese received for each recorded follow-up in the PMDA tab. The system displays a list of all follow-ups that are entered in the General tab with the option to specify Japanese received for each follow-up entry.
- When you change the Japanese Received Date from default, the justification pop-up with the message Please enter the reason of information receipt date change is displayed, wherein you are required to enter the reason of this date change.
- When you change the Japanese Receipt Date or Japanese Follow-Up Date, if the changed date is older than the date in the Information Receipt Date of General tab, a pop-up window with the following error message is displayed:
The date cannot be older than the first information receipt date of the case.
- For the purpose of Report Scheduling in Japan, the Japanese Aware Date is used by checking the flag on Use Japanese Aware Date for Reporting as configured in the Reporting Destination Code List of Console. The Japanese Aware Date has the same behavior as the Standard Aware Date.
-
Reporting Category:
The Reporting Category is a drop-down list with the values from Reporting Category Code List.
- The Reporting category is a drop-down list with the values, as indicated in the table below. The drop-down content of the field depends on the previous selection in:
- AE/Infection (in Infection check box in Event tab) - This is used only if all the events are AE, or all the events are Infection.
- Domestic / Foreign (in Country of Incidence, PMDA tab, Dom = JP, Frgn <> JP)
- License Type (Investigational or Marketed)
- You can refer to the following table for the list of Reporting Categories in the drop-down list.
- The system clears the Japan Reporting Category if the dependent fields are modified after the selection has been updated.
Table 1-2 List of Reporting Categories
R2 Category R3 Category Category Description AE/ Infection (in event tab) Dom / Frgn License Type Paper Reports A
AA
Case reports on infections in Japan (post-marketing)
Infection
Dom
Mkt
Form 1, 2
(6 pages)
B
AB
Case reports on adverse drug reactions in Japan (post-marketing)
AE
Dom
Mkt
Form 1, 2
(6 pages)
C
AC
Case reports on infections in foreign countries (post-marketing)
Infection
Frgn
Mkt
Form 1, 2
(6 pages)
D
AD
Case reports on adverse drug reactions in foreign countries (post-marketing)
AE
Frgn
Mkt
Form 1, 2
(6 pages)
E
AE
Research reports on infections (post-marketing)
Infection
Any
Mkt
Form 3, 4
(2 pages)
F
AF
Research reports on adverse drug reactions (post-marketing)
AE
Any
Mkt
Form 3, 4
(2 pages)
G
AG
Reports on corrective action such as discontinuation of manufacturing, recall, disposal in foreign countries (post-marketing)
Any
Any
Mkt
Form 5, 6
(2 pages)
H
DA
Case reports on infections in Japan (clinical trial)
Infection
Dom
Inv
Form 1, 2
(6 pages)
I
DB
Case reports on adverse drug reactions in Japan (clinical trial)
AE
Dom
Inv
Form 1, 2
(6 pages)
J
DC
Case reports on infections in foreign countries (clinical trial)
Infection
Frgn
Inv
Form 1, 2
(6 pages)
K
DD
Case reports on adverse drug reactions in foreign countries (clinical trial)
AE
Frgn
Inv
Form 1, 2
(6 pages)
L
DE
Research reports on infections (clinical trial)
Infection
Any
Inv
Form 3, 4
(2 pages)
M
DF
Research reports on adverse drug reactions (clinical trial)
AE
Any
Inv
Form 3, 4
(2 pages)
N
DG
Reports on corrective action such as discontinuation of manufacturing, recall, disposal in foreign countries (clinical trial)
Any
Any
Inv
Form 5, 6
(2 pages)
O
BC
Research reports on quasi-drugs
Any
Any
Any
Form 3, 4
(2 pages)
P
BD
Research reports on cosmetics
Any
Any
Any
Form 3, 4
(2 pages)
- The Reporting category is a drop-down list with the values, as indicated in the table below. The drop-down content of the field depends on the previous selection in:
-
PMDA Tab > General > Linked Product section collects product in the Assessment table with the following rules:
- System checks:
- If the case is a foreign case
- If the case has non-company product(s).
- The non-company product(s) is/are marked as Suspected.
- Under all above conditions, suspected company product in this case has equivalent Japanese license.
- If there is non-company suspected drug in foreign cases, that product Trade Name and Generic name are used for matching with the Keywords from Console J > Code List > Argus J > Reportable Product Keyword.
- This keyword is used for finding related company product family which needs reportability assessment when foreign case has non-company suspected drug. The keyword text is matched within either the Product Name or Generic Name fields of the Case Form. If entered keyword matches (partially or fully), the matching product family's Japanese license is assessed in the PMDA tab.
- When there are multiple Japanese licenses in the matching family, all of these licenses are listed for assessment in the PMDA tab.
- Products already existing in the Case form are not populated here.
- In this section, the Causality Assessments are available.
- Listedness is always Unknown for this section.
- Hyperlinks are not available for this Assessment
section.
Table 1-3 Field Descriptions
Field Argus J User display Product
Company product(s) using J Reportable Keyword are displayed.
Event PT (Description) / LLT
Event(s) in the case are displayed
D/S
D/S information is displayed as read-only
SeriousnessSeverityDuration
Event information is displayed as read-only
Reported Causality
Reported Causality type-ahead drop-down
Determined Causality
Determined Causality type-ahead drop-down
- You can assess the product for reportability in this table.
- This section's display status (Maximize/Minimize) is not dependent on a user preference:
- This section is automatically minimized if there is no data.
- This section is automatically maximized if there is data.
- System checks:
-
When you enter future date in Initial Receipt Date in Case Form > General tab > General Information > Initial Receipt Date, or PMDA Tab > General > Initial Information Receipt date, the application displays the following validation message:
Receipt Date cannot be a future date
-
Product Change Justification:
- When you click the OK button (the button appears in each follow-up row of Product Change Justification column in the follow-up info table), the Justification Table pop-up is displayed to view update information.
- The button is enabled only when Product Change justification exists for the case.
For example, Scenario # 1 - it only asks for justification if there is a submitted report against the product which is subject to deletion and you unlock the case with follow-up to delete that product.
Scenario # 2 - In addition, when you create a company product:
- Schedule an Expedited report against the company product.
- Get that report to Submitted status.
- Unlock case a, add follow-up and change the company product to a different company product (change justification is displayed).
- Enter something and navigate to PMDA tab.
- The button is enabled.
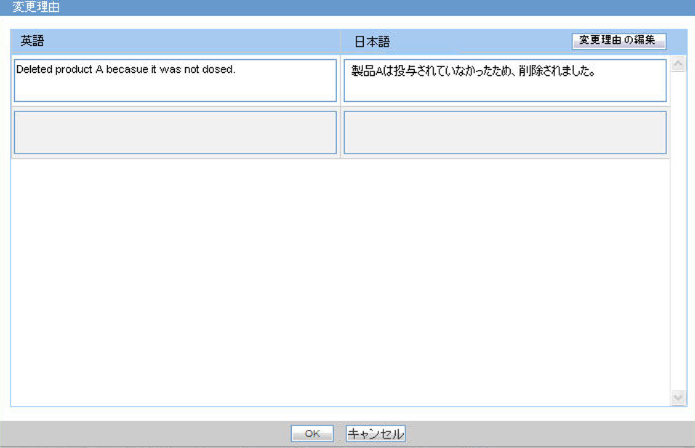
- The Justification list populates all the product justifications entered for the follow up.
- The Translation justification is editable using the following steps:
- Select the justification from the list. This highlights the row.
- Click the Editing the justification button.
This opens Justification pop-up and you can edit the justification.
-
When you select the Research Report Type Reporting category, a pop-up Reason for subject of the Research Report is displayed to enter the reason of the Research report.
- The pop-up UI section opens only when any one of the suspected drug license table
has one of following Reporting Category
selected:
Table 1-4 Reporting Categories
# Reporting Category (English meaning) 5
Research/Infection report (Marketed drug)
6
Research/ADR report (Marketed drug)
12
Research/Infection report (Investigational drug)
13
Research/ADR report (Investigational drug)
15
Research report (Quasi drug)
16
Research report (Cosmetics)
- The information is multiply stored when multiple suspected licenses with the Research Reporting Category exist. Each Research Reporting Category has a circle in Green color symbol after the justification is entered in the pop-up window.
- Justification pop-up
- User Interface:
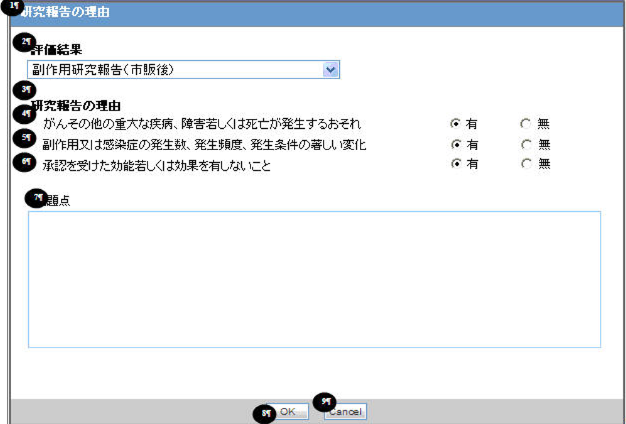
The following table explains the fields used in the Justification dialog box:
# Field Label Value English Label Name Purpose Field Length Audit Log 1
N/A
Reason of Research Report
Title of the UI
N/A
N/A
2
N/A
Assessment Result
Read Only
N/A
N/A
3.
N/A
Reason of Research Report
Title of the section
N/A
N/A
4.
Reason for subject of the Research Report - Possibility of occurrence of serious disease such as cancer, disorder, or death
Possibility of occurrence of serious disease such as cancer, disorder, or death
Radio button Yes - exists
無No - does not exist
N/A
Yes
5.
Reason for subject of the Research Report - Significant change on event or infection occurrence number, frequency, and condition
Significant change on event or infection occurrence number, frequency, and condition.
Radio button Yes - exists
無No - does not exist
N/A
Yes
6.
Reason for subject of the Research Report - It doesn't have acknowledged effectiveness
It doesn't have acknowledged effectiveness
Radio button Yes - exists No - does not exist
N/A
Yes
7.
Reason for subject of the Research Report - Problems
Problems
Text Field, 20,000 field length.
20,000
Yes
8.
N/A
OK
Button
N/A
N/A
9.
N/A
Cancel
Button
N/A
N/A
Items # 4, 5, and 6 have radio buttons Yes and No to store the answer.
Field #2, Assessment Result, is a read-only field to show either the value from Literature Intake Assessment or the Reporting Category selected in the PMDA General tab. The following table illustrates the drop-down content based on the Reporting Category:
# Drop-down Content (English meaning) Reporting Category 1
Not Necessary
NA
2
AE Case
A, B, C, D, H, I, J, K
3
Research/Infection report (Marketed drug)
E
4
Research/ADR report (Marketed drug)
F
5
Research/Infection report (Investigational drug)
L
6
Research/ADR report (Investigational drug)
M
7
Research report (Quasi drug)
O
8
Research report (Cosmetics)
P
9
Measures in foreign countries including discontinuation of manufacture, recall and withdrawn (Marketed drug)
G
10
Measures in foreign countries including discontinuation of manufacture, recall and withdrawn (Investigational drug)
N
- User Interface:
- The Problems section text field stores text information.
- At least one Yes radio button has to be filled in the User Interface, and if this condition suffice, the UI can be closed. If this is not fulfilled, a message:
At least one question has "Yes" selected is displayed to suffice the condition.
- This is not printed in the Case Form Print.
- Once you select Research Reporting Category and enters the reason, and then re-selects the Reporting Category to Non-Research Reporting Category, the system displays a warning message:
Trying to select non-Research Reporting Category. If changed, the already entered "Reason of the Research Report" will be removed. Continue? is displayed.
If you select OK, the reason is deleted. If Cancel is selected, the Reporting Category change is aborted.
- The pop-up UI section opens only when any one of the suspected drug license table
has one of following Reporting Category
selected:
-
Each section has the Minimize button to hold the sections. The Associated Product Assessment section is minimized when the product does not exist.
-
Based on the TIKEN configuration in the console, if the license is available in PMDA Info > General > Suspect Product Licenses and it is a foreign case (that is, COI is other than Japan) then the TIKEN icon is displayed in the Product Name and License Number column as follows:
Parent topic: PMDA Information tab