Products Tab
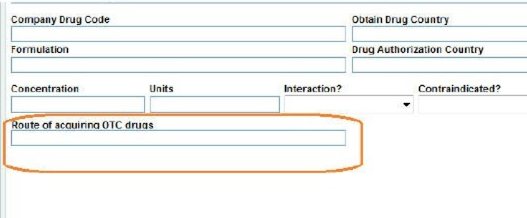
Route of acquiring OTC field
The Route of acquiring OTC drugs field under Product Information captures the route of acquisition of the company's own products in the form of pre-defined PMDA specified code list values, when it is an OTC drug, and it is transmitted in the J2.6k element of the PMDA E2B (R3).
This field is available in the J view only to the local user for the local data entry after a global lock. This field is enabled only when the product is marked as suspect, and there is at least one Japan license in the case for which the OTC Product flag in the License Configuration is marked as checked.
If the product category (for both blinded or broken blind cases) is changed between Suspect / Concomitant / Treatment or Product license are re-evaluated (from the Event Assessment tab), the enabling criteria for this field is also re-evaluated based on the latest case data.
J Drug Code field
You can modify the J Drug Code for a company product and retain the product as a company product.
The corresponding English product name for a J Drug populates (only if it is blank):
- When you encode a drug using the J Drug Browser from Case Form > Products tab (Japanese) > Encode button into Case Form > Products Tab (English) > Product Information > Product name using the English Drug Name value that is loaded in the J Drug Schema using the English sub file.
- When you add the J Drug to the Case Form > Patient/Parent > Other Relevant History section into the corresponding English field in the Case Form.
- When you add or select a study drug, which is a J Drug configured from Console > Business Configuration > Study Configuration (J pop-up), in Case Form > Product name.
- When you select the Study Drug (in a Case Form for a Study case) as J Drug (as configured in the study configuration), into the English version of the Case Form only if it is a not blinded study (as it does for the existing products).
- In a globally locked and locally unlocked case if you encode a non-company product with the J Drug using the J Drug Browser into the corresponding English Drug Name shall is not updated in Case Form > Product Name.
The corresponding English product name is not updated in the English version of the Case Form > Product name, in a globally locked and locally unlocked case when you encode a non-company product with the J Drug using the J Drug Browser.
J Drug Code under Substance Information section
The J-DRUG field has been added in Case Form > Product > Substance Information.
This field populates the J Drug Code of the Ingredient as configured in Argus Console > Business Configuration > Product and Licenses > Product (J Data Entry) > Key Ingredients and it is editable.
Device subtab
The Device Information section on Case Form > Products tab > Device sub tab contains the following fields as required for the PMDA Device XML and Paper reporting for Form 8 and Form 10:
- Device Outcome
- Device Outcome Details
- Device Comments
The Health Impact Information section, the Device Problem Information section, the Evaluation / Investigation Code Information section contain the following fields as required for the PMDA Device XML and Paper reporting for Form 8 and Form 10:
- Causality
- Listedness
- Suspicion or Risk
- JFMDA code
The Malfunction Information section is available under Case form > Products > Device (Tab) > Device Information. This section is not used in the PMDA Device XML reporting and updated Paper form reporting for Form 8 and Form 10.
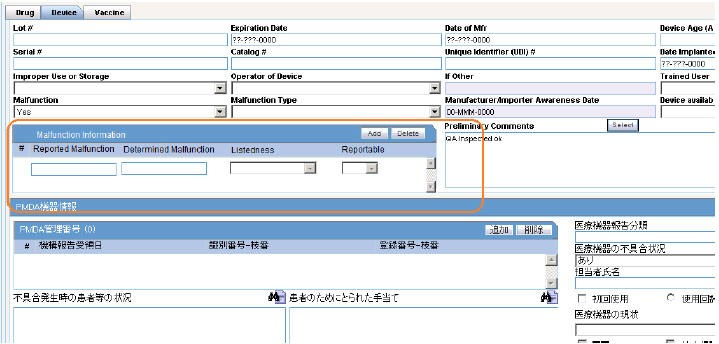
The Malfunction Information section contains the following fields:
| Field | Description |
|---|---|
|
Reported Malfunction |
Enter the malfunction name as reported by the reporter. |
|
Determined Malfunction |
Enter the malfunction name as determined by the company. |
|
Listedness |
Select the listedness of malfunction in respect of the device. |
|
Reportable |
Select the reportabilty of the malfunction. |
|
Add |
Add a new blank row to the table. This button is always enabled. You can add up to 50 rows using this button. If the number of rows exceeds 50, when clicking the Add button, an error message is displayed: When this window is reloaded or refreshed, the empty rows are not displayed. The first row is highlighted in light yellow (selected) by default as a standard Case Form behavior. |
|
Delete |
Delete a row from the table. This button is always enabled. To delete a row, select that row and click Delete. A warning message is displayed: |
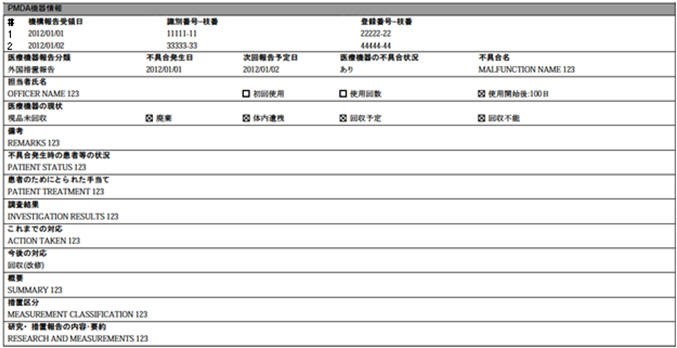
The PMDA Device Information section in Case Form > Products tab > Device subtab contains fields as specified in the following image:
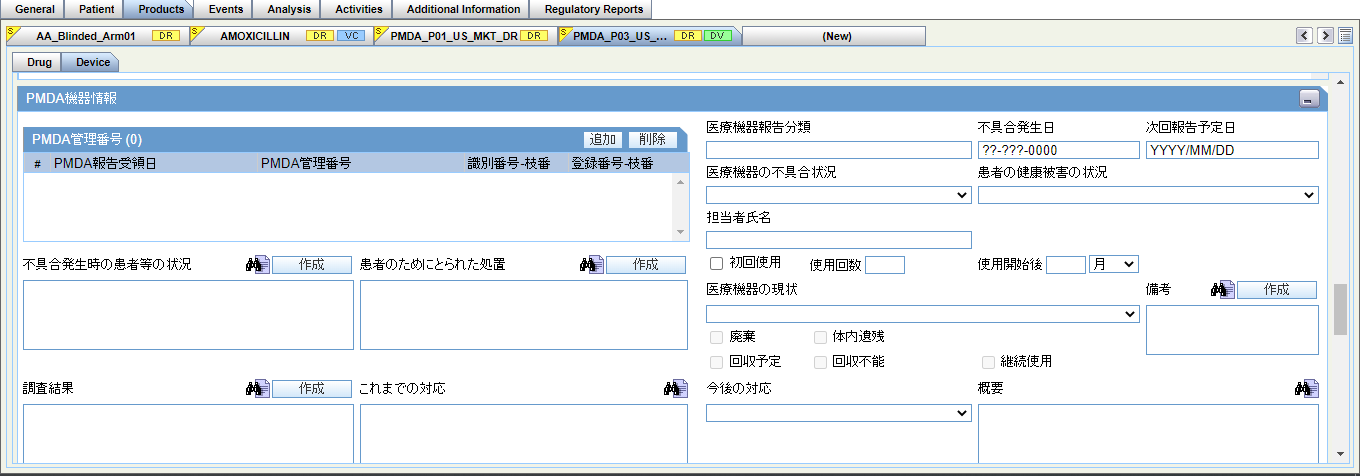
The PMDA Information section contains the following fields as required for PMDA Device XML and Paper reporting for Form 8 and Form 10:
- PMDA Control Number
- Nullification Reason
- Nullification Reason Details
- Complete
- Reason for Incompletion
- Future Response
- Corrective Action Category
- Usage continuing
The Status of Patient's health damage field has been added under Case form > Products > Device (Tab) > PMDA Device Info. This drop-down list has the values <blank>, Exists, Does not exist and Unknown.
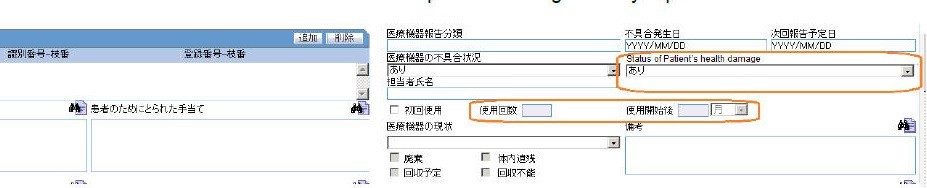
You can select any value in PMDA Device Info > Medical Device Reporting Category, irrespective of the Device Information > Malfunction value. This implies that irrespective of the value selected in the Malfunction drop-down list, you can select all the possible values under Medical Device Reporting category.
You can enter values in First time use, Times of use and Since the first use fields at the same time, independent of each other.
The PMDA Device Information section is available to all Oracle Argus Safety Japan users on both English as well as Japanese views as uncollapsed by default only when the Japanese module is enabled. It is displayed with Japanese labels on both English as well as Japanese views as this section is not meant for translation of data. The Modify, View and No Access rights to this section are based on the Product Information (Device View) option in Console > Access Management > Groups > Case Form section. The tabbing order of the case form elements respects this section in the order of the UI elements as left to right and top to bottom.
The PMDA Device Information check box in the Case Print - section selection dialog is available only to the Oracle Argus Safety Japan users:
- This check box is added right after MedWatch Device Information option in the Case Print options dialog box. All the options after it are shifted further by one place.
- This check box gets selected and unselected when user uses Select All and Unselect All options.
- This check box remains unchecked by default and is disabled unless its parent
section check box, Product Device Information, is checked by the user. If Product
Device Information is unchecked later, then PMDA Device Information also gets
unchecked and disabled. The Case Form Print PDF report prints this section in
Japanese after the MedWatch Device Information section if it is selected for
printing in the section selection dialog box.
- The Case Form Copy function copies this section when a case is copied with this section checked in the Case Copy options dialog box.
All fields of this section are audit logged as other case form product device fields.
All fields of this section are available under Console > System Configuration > Field Labels, Field Validations, and Advanced Conditions screens under the tree structure Argus Safety > Products > PMDA Device. These fields are not required for Aggregate Reports.
In Console System Configuration Field Labels screen, all these fields are available with the following attributes:
- Hidden radio option set to No. Hiding is allowed.
- Read-Only options have been unchecked and disabled.
- E2B Field and Research Field are unchecked by default.
Parent topic: Case Form