- User's Guide
- Oracle Argus Affiliate Users
- Entering Event Information
Entering Event Information
- Click Add to add another row to the section.
- Click the Zoom icon to enter text or notes in a separate window. You can also check the spelling of the text in this separate window.
- Open the AE Entry tab.
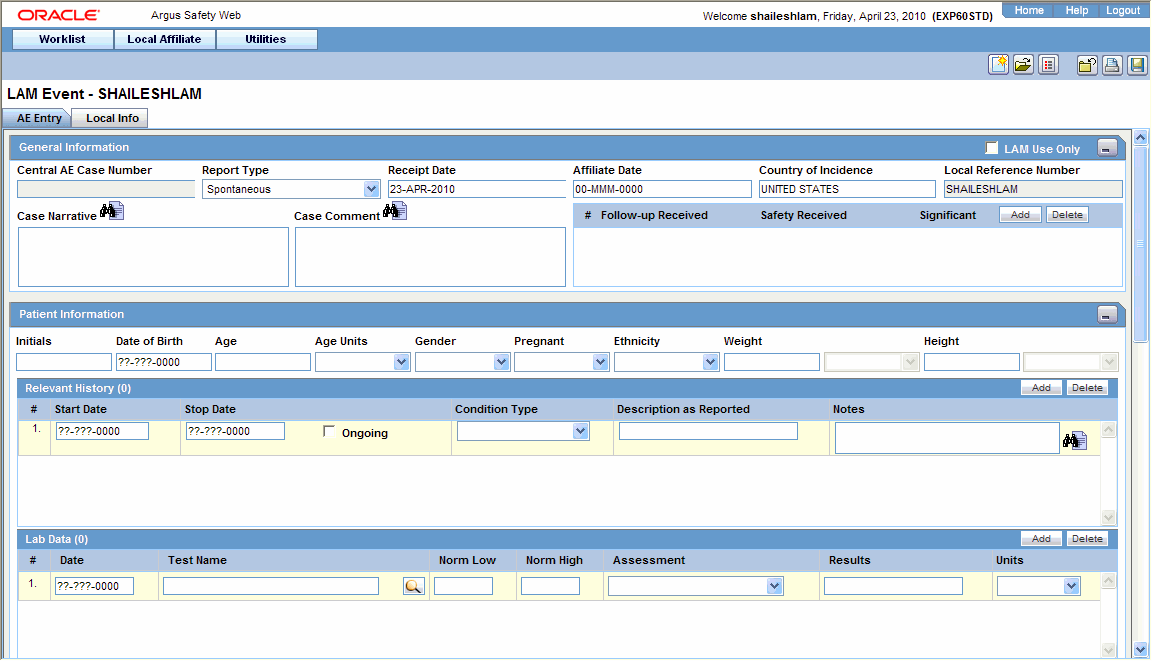
- Enter the available event information in each of the sections of the AE Entry tab as shown in the following tables.
General Section
Item Description LAM Use Only
Select this check box if this event is for local usage only and will not be routed to Central.
Central AE Case Number
The case number that is assigned to this event at Central will be entered here. No text can be entered in this field when initial event information is being entered.
Report Type
Select the type of report.
Receipt Date
Enter the Receipt Date. This date will be used at the Central end.
Affiliate Date
Enter the date received.
Country of Incidence
Select the country in which this event occurred.
Local Reference Number
The number by which the event is identified is entered here. This number will be automatically generated if the system has been configured to automatically number local events.
Case Narrative
Enables the user to enter a narrative description of the case.
Case Comment
Enables the user to enter relevant comments about the case.
Follow up Received
Enter the date on which follow up information was received at the local affiliate location.
Safety Received
Enter the date on which the follow-up information was received by the Central safety office.
Significant
Select this check box if significant follow-up information has been received.
Patient Information Section
Item Description Initials
Enter the initials of the patient.
Date of Birth
Enter the date of birth of the patient.
Age/Age Unit
Enter the patient's age and the age units used.
Gender
Enter the patient's sex.
Patient ID
Enter the patient's identification number. Note: This field is available only if the event is associated with a clinical trial or other study.
Study Number
Enter the study number. Note: This field is available only if the event is associated with a clinical trial or other study.
Other Relevant History Section
Item Description Start Date
Enter the start date of the condition. You can enter a partial date if the actual date is not available. You can also choose not to enter a date.
Stop Date
Enter the stop date of the condition. You can enter a partial date if the actual date is not available. You can also choose not to enter a date.
Ongoing
Indicates whether the condition is continuing. If it is set to Yes or Unknown, the Stop date is disabled.
Condition Type
Select a condition type from the list. The Administrator can adjust this list.
Description as Reported
Enter the verbatim term used by the reporter, or patient, to describe the adverse event or product name.
Notes
Enter any notes pertinent to relevant history.
Reporter Information Section
Item Description Address
The address of the institution.
City
The city where the institution is located.
Country
The Country where the institution is located.
Department
The name of the reporter's department
Email Address
The email address for the reporter.
FAX Number
The fax number for the reporter.
First Name
The first name of the reporter.
Health Authority Case Number
The Health Authority Case Number.
Health Care Professional
Indicates whether the reporter is a health care professional.
Institution
The name of the institution where the reporter is currently employed.
Intermediary
Identifies the intermediary for the case.
Last Name
The last name of the reporter.
Middle Name
The middle name of the reporter.
Occupation
Displays the occupation of the reporter.
Postal Code
The postal code where the institution is located.
Protect Confidentiality
Indicates that the reporter's identity is protected in expedited reports.
Dosage Regimen Dose Description
User this field to describe non-standard dosages that cannot be adequately described using the dose, route, and frequency fields. Note that the initial value for this field defaults to the dose, route, and frequency information and can be amended as appropriate.
Report Media
Displays the method used to report the case.
Reporter Type
Identifies the reporter type.
Sal.
Identifies the title of the reporter.
State
The state where the institution is located.
Suffix
Displays the reporter's suffix, if appropriate.
Event Information Section
Item Description Onset Date
Enter the date/time the event started. You can enter a partial date if the complete date is unavailable.
Stop Date
Enter the date/time the event stopped.
Event Description
Enter the verbatim term used by the reporter to describe the adverse event. As you type, the system automatically copies the term in to the Description to be Coded field.
Death
Click the appropriate box to select the Serious Criteria for the event.
Hospitalized
Click the appropriate box to select the Serious Criteria for the event.
Disability
Click the appropriate box to select the Serious Criteria for the event.
Other
Click the appropriate box to select the Serious Criteria for the event.
Other Text
Enter text to describe the other type of serious event.
Medically Significant
Click the appropriate box to select the Serious Criteria for the event.
Life Threatening
Click the appropriate box to select the Serious Criteria for the event.
Intervention Required
Click the appropriate box to select the Serious Criteria for the event.
Congenital Anomaly
Click the appropriate box to select the Serious Criteria for the event.
Symptoms
Enter the appropriate symptoms for the cases.
Outcome
Select the outcome of the event from the drop-down list (e.g., recovered, improved, fatal, etc.) The Administrator can adjust the information on this list. If Fatal is selected, Death is check in the list of seriousness criteria.
Duration
The system automatically calculates this field from the event start and stop dates. If duration is greater than five (5) days, the system only displays days. You can enter or modify the duration manually.
Diagnosis/Symptom
Click the appropriate radio button to indicate whether the event is a diagnosis. Clicking Yes marks this event as the primary event. In case of multiple diagnosis events, the event on the left is considered as the primary event. Click Relationships to display the Event-Relationships dialog. This enables you to group symptoms and signs with diagnoses.
- Open the Local Info tab.
- Enter the available case information in each of the sections of the Local Info tab as listed in the following tables.
Contact Log Section
Item Description Date
Enter the date associated with the letter.
Code
Enter the contact code associated with the letter.
Description
Enter the description associated with the letter.
User
Select the user to whom the letter is to be sent.
Date Sent
Enter the date on which the letter was sent.
Action Items Section
Item Description Date
Displays the date associated with the Action Item.
Code
Displays the code associated with the Action Item.
Description
Displays a description of the Action Item.
User
Displays the user to whom the Action Item is assigned.
Due Date
Displays the date on which the Action Item is due.
Date Completed
Displays the date on which the Action Item was completed.
Notes and Attachments Section
Item Description Attach Documentum Link
Displays the Documentum Lookup Dialog. Use this dialog to search for and select Documentum links.
Attach File
Click Attach File to add an attachment. Browse to the location of the file on your system.
Date
Enter the date associated with the note or attachment.
Classification
Select the type to which the attachment belongs.
Description
Enter the description of the attachment.
Item
Description
Keywords
Enter the keywords relevant to the attachment. To select a keyword from a list, click Select.
Select
Click Select to enter a new keyword or select a keyword from the list.
Routing Comments Section
Item Description Date
Displays the date on which the event information or follow-up information was routed to Central.
Route By
Displays the user who was responsible for the routing.
Comment
Displays the routing comments.
- Add letters as necessary. Select Save in the
Local Affiliate menu to save the case.
Note:
For follow-up information associated with an event, enter the follow-up information and then route the event to Central.
For more information, see:
Parent topic: Oracle Argus Affiliate Users