Common Considerations for Coding Information
Consider the following points to add or update the coding information for Medical Device Problem, Health Impact, Clinical Signs, and Evaluation/Investigation:
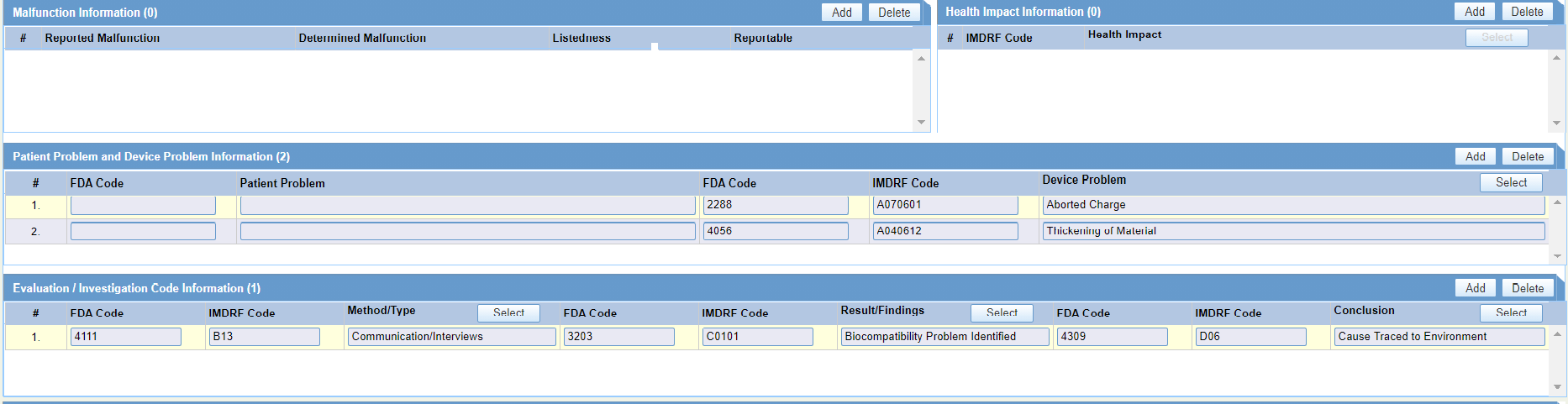
- Entry of Coding Information is done only by invoking the look-up, New Look Up will have both FDA and IMDRF codes for Medical Device Problem Coding, Evaluation/Investigation Coding, Health Impact Coding, Clinical Sign Coding and Device Component Coding.
- For a case which already has Medical Device Problem and Evaluation Codes and if an obsolete FDA code is used in the case, the decode values should not be displayed in Device Problem and Evaluation/Investigation Result, Method and Conclusion. Coding User/Data Entry User is expected to recode such data against the new repository.
- For Legacy cases, you are expected to recode the Medical Device
Information and Evaluation/Investigation
Information to populate IMDRF Codes based on FDA codes.
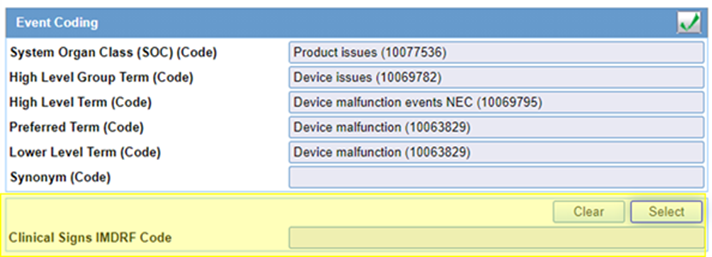
- The Clinical Signs IMDRF Code field is added in the
Event tab, System supports a look up to select the IMDRF
Code for the Coded Event, system populates the English MedDRA LLT Term of the Event
in the Term text box of the Search window to support Clinical Sign Coding, After the
selecting the Clinical Sign Term, system displays the Clinical Sign Term and IMDRF
Code in the IMDRF Code field
Example: Balance Problems (E0101).
Parent topic: Update Device Tab within the Product Tab