Update Device Tab within the Product Tab
Important Data Entry considerations for various fields in the Product Tab are listed below.
Note:
Fields that have direct mapping and do not have validation implications are not treated here.| Field Label | Date Entry Consideration |
|---|---|
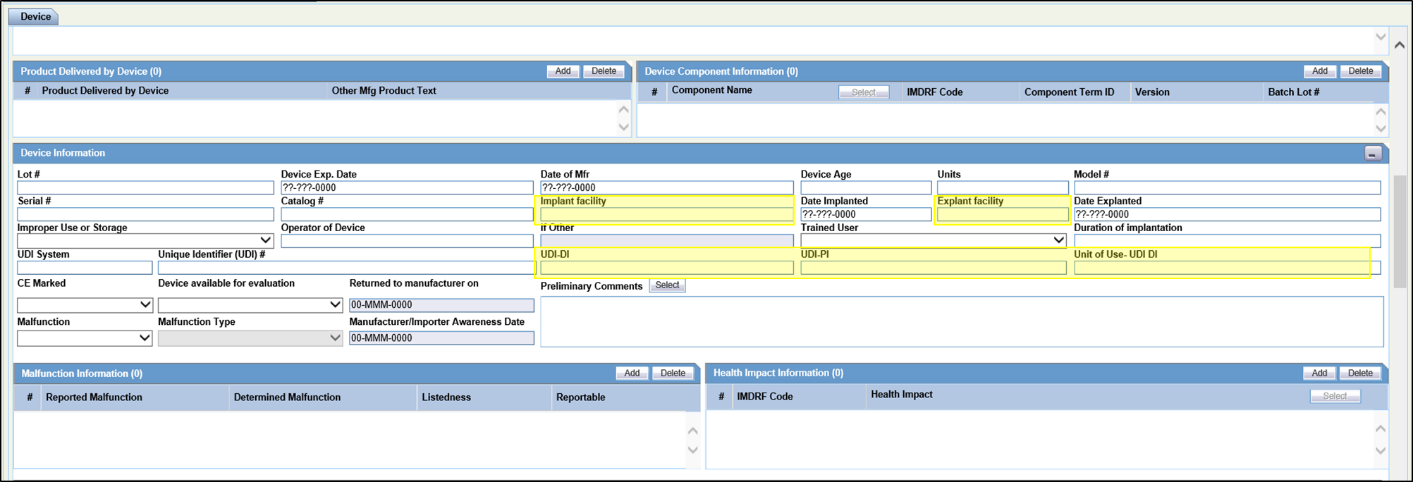
| UDI-DI | EC recommends that for Products which are classified with Risk Class of the device as IVDR and MDR, the UDI-DI field is mandatory.
It is recommended that Manufacturer updates the UDI-DI in the case form with products with Medical Device Information configured with IVDR and MDR values. |
| UDI-PI | EC recommends that for Products which are classified with Risk Class of the device as IVDR and MDR, the UDI-PI field is mandatory.
It is recommended that Manufacturer updates the UDI-PI in the case form with products with Medical Device Information configured with IVDR and MDR values. |
| Preliminary Comments | This field captures the Preliminary results and conclusions of manufacturer’s investigation. This field is mandatory for Initial and Follow up MIR Reports. It is recommended to include this as Page 13 of 22 part of the data entry guideline so that the required data is populated in the MIR Initial and Follow-up Reports. |
| QC Result | This field captures the Manufacturer’s evaluation of the Incident, This field is mandatory for Final and Combined Initial & Final MIR Reports. It is recommended to include this part of the data entry guideline so that the required data is populated in the MIR Final and Combined Initial & Final Reports. |
| Implant Duration | If Implant and Explant dates are not known, it is important for the Manufacturer to provide information on approximate implant duration of the Device; It is recommended to enter data in the below format:
|
For more information, see: