4 Configuration updates in Argus Console
The following section describes the updates in Argus Console for the PMDA Device Form 8 and Form 10 reports in XML format and paper forms support.
Device Reporting Category
The old factory data for the Device Reporting Category values are updated to set the Display value to No. It is recommended that you do not modify the display settings since the old values are no longer accepted by PMDA.
| Device Reporting Category | Device Reporting Category (J) | Display |
|---|---|---|
|
Infection Report |
感染症報告 |
No |
|
Malfunction with health damage |
副作用報告 |
No |
|
Malfunction without health damage |
不具合報告 |
No |
|
Measures in Foreign Country Report |
外国措置報告 |
No |
|
Research Report |
研究報告 |
No |
Common Profile Switches
- JPEG
- JPG
- BMP
- PNG
- GIF
- TIF
- TIFF
- RTF
- TXT
- XLS
- XLSX
- DOC
- DOCX
- XML
- DICOM
- HTML
Note:
It is recommended to review the supported attachment formats and remove formats that are not required as per the business need.However, Argus does not support uploading HTM or HTML file types. It is recommended that you do not upload/attach HTM/HTML files.
PMDA Device Profile
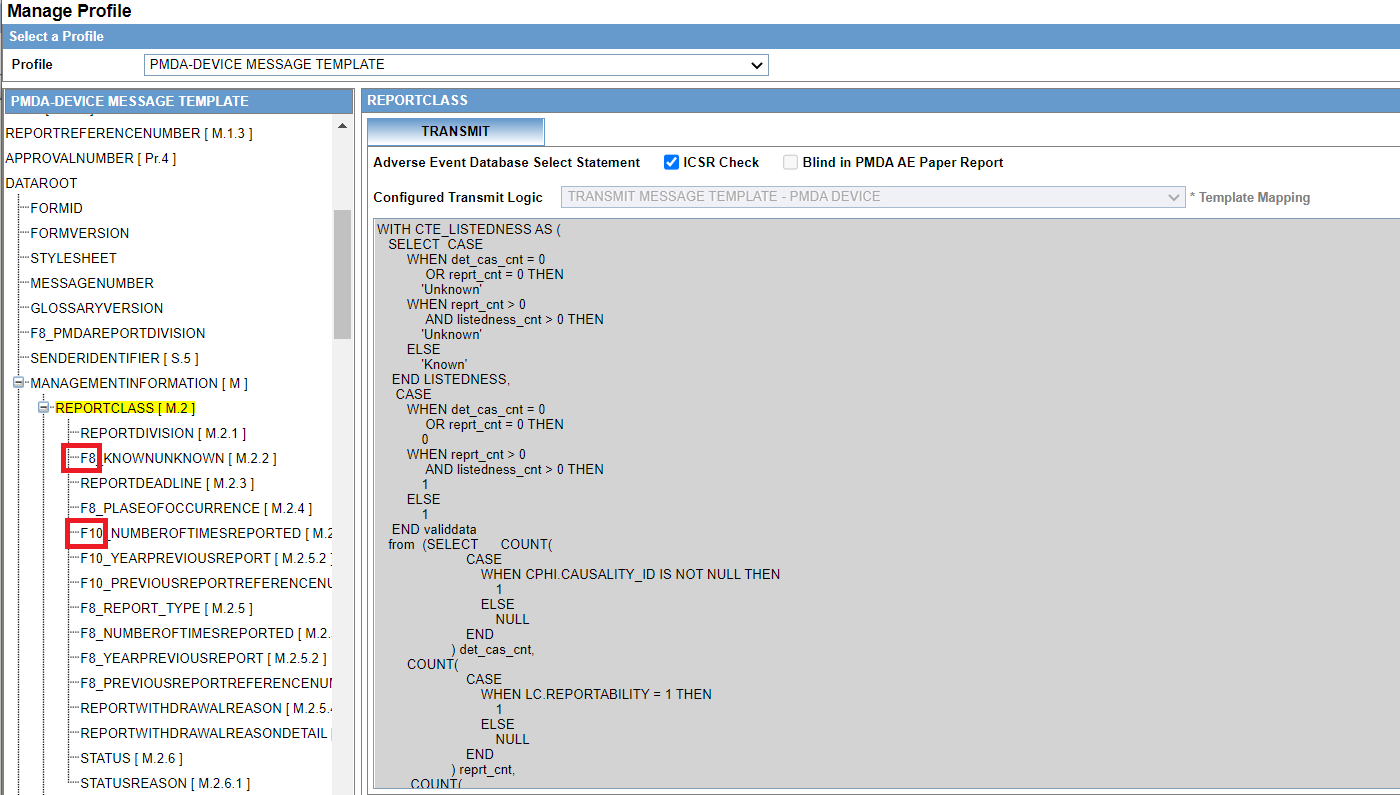
- The standard device profile, PMDA-DEVICE MESSAGE TEMPLATE, uses a similar framework as the E2B(R3) profile.
- Based on the reporting category selected in case form, either Form 8 or Form 10 XML is generated.
- You can customize the transmit logic for each of the elements by copying the profile and changing the SQL query similar to E2B(R3) profile. Since the profile is common for Form 8 and Form 10 report, the customization logic caters to both report forms.
- For example: If you update the logic for element DOCUMENT for compression logic, the compression logic is applied to both Form 8 and Form 10 reports attachments.
- Since most of the data elements with respect to Form 8 and Form
10 device XML are same, the profile has elements related to both Form 8 and
Form 10 XML reports. To distinguish elements specific to Form 8 and Form 10,
elements are pre-fixed with the F8 and F10 tags, as shown in
the screenshot below:
Figure 4-1 Manage Profile
Product Configuration
PMDA has published the Medical Device Defect glossary containing the JMDN and JFMDA details. The JFMDA codes are mapped to IMDRF codes. The glossary is loaded as factory data into the Oracle Argus database.
It is recommended that you do not update the glossary from the backend.
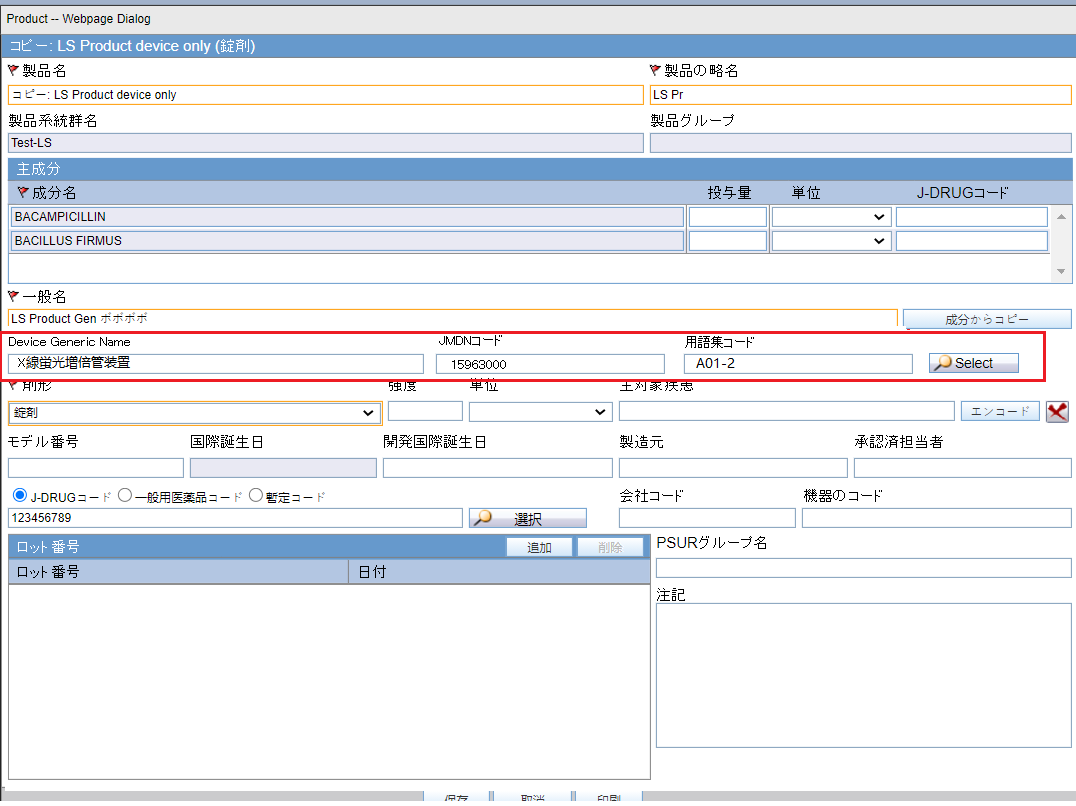
- The device generic name, along with the corresponding JMDN code and terminology code can be configured in the J Data Entry pop-up window. To access it, select the device from the Look up window which lists the JMDN glossary loaded into Argus system.
- You can manually type the device generic name, if the device is not currently part of glossary. Allowing manual entry is implemented based on the feedback from customers. The Oracle Argus system directly transmits whatever is configured in Argus Console. However, PMDA has a validation check for the generic name and JMDN code to be part of glossary. So, if you are manually entering these fields, it is recommended that you work with PMDA to accept the generic name and JMDN code.
- If the terminology code is not assigned for any of the selected or entered device generic name, then you can use the Z01 code for common terms.
- If the product being configured is purely a device product (not a combination product), the device generic name you select in the J Data Entry pop-up window can be configured as a Generic name for the product
Figure 4-2 Product Configuration