Example: Adverse Events and Concomitant Medications association
This example shows the report output for the association between an adverse event on the Adverse Events form and a drug on the Concomitant Medications form for subject PRE.
In this example, there is a one-to-one relationship between a single adverse event and a single concomitant medication for the subject. If, for example, the subject reported a second adverse event associated with the drug Anacin, an additional row would appear in the report listing the second Adverse Event (in the Event column), and Anacin listed in the Drug name column.
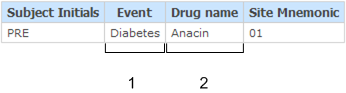
- 1—(Points to the Event column.) The Event column contains adverse event data for each subject.
- 2—(Points to the Drug name column.) The Drug name column contains concomitant medication data for each subject.
Parent topic: Data from associated forms