12 About study level submissions
Note:
This section describes how to work on study level submissions (a.k.a. EU submissions) in Oracle Site Activate. If you need information about working on regular, non-EU submissions please refer to About submissions instead.Create and manage study level submissions
Oracle Site Activate supports submissions to the European Union (EU) Clinical Trials Information System (CTIS). Supporting features include the definition of study level submissions and packages, the ability for one EU member state to submit on behalf of other EU member states, as well as use of activity level due dates.
EU Submissions you to manage study level submission packages that have been deployed to Oracle Site Activate with one of the following activity types:
- Define EU Submission Validation Package
- Define EU Submission Part 1
- Define EU Submission Part 2
Validation, Part 1, and Part 2 submission packages each have unique package definition requirements, and Oracle Site Activate includes definition modals that provide all appropriate fields for completion.
After study level submissions have been defined for your study, you'll see the study level submission milestones on the study and site level milestone timelines. Additionally, study level submission milestones displayed on countries and sites include the associated study submission or submission package as a read-only item.
Defining an EU submission validation package
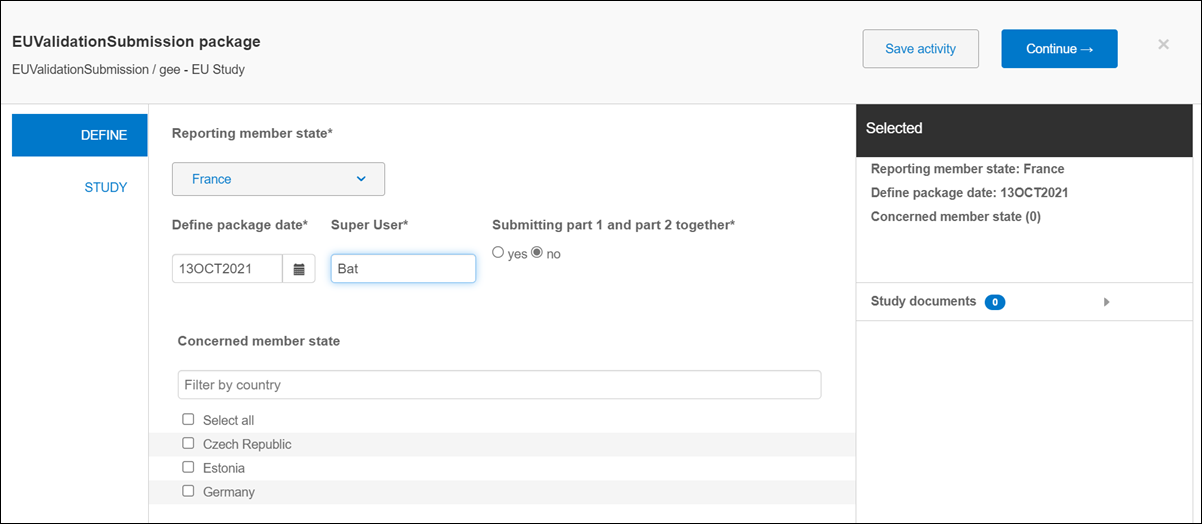
When you open a study level submission modal and click “Plan the Submission Package,” the planning modal opens the validation package’s define step, where you'll enter or select values for the following fields:
- Reporting Member State (the drop-down list includes the 27 EU Member countries)
- Define Package Date
- Super User
- Submitting part 1 and part 2 together (Yes / No option)
- Concerned member state (with a search field, multiselect, and Select All options)
As you define the Reporting member state, Define Package Date, and Concerned member state(s), the modal’s “Selected” panel updates with your selections.
The definition modal allows multiple users to define the package. When a group of users works together to define the validation package, any user can click “Save activity” at any time to save their progress without completing the activity, and that user can return to the package modal later to finish package planning. When multiple users are planning the package, they’ll also see the name of the user who last made updates displayed in the Selected panel.
When a user clicks the modal’s Continue button, the modal saves and automatically advances to the Study document selection area. As the user chooses individual study-level documents, the Selected panel also updates to reflect the chosen documents.
When a user clicks the package’s “Done” button, a confirmation request displays, and when confirmed, the activity is completed, and the completion date is the date selected from the “Define Package Date” date picker.
Defining an EU submission Part 1
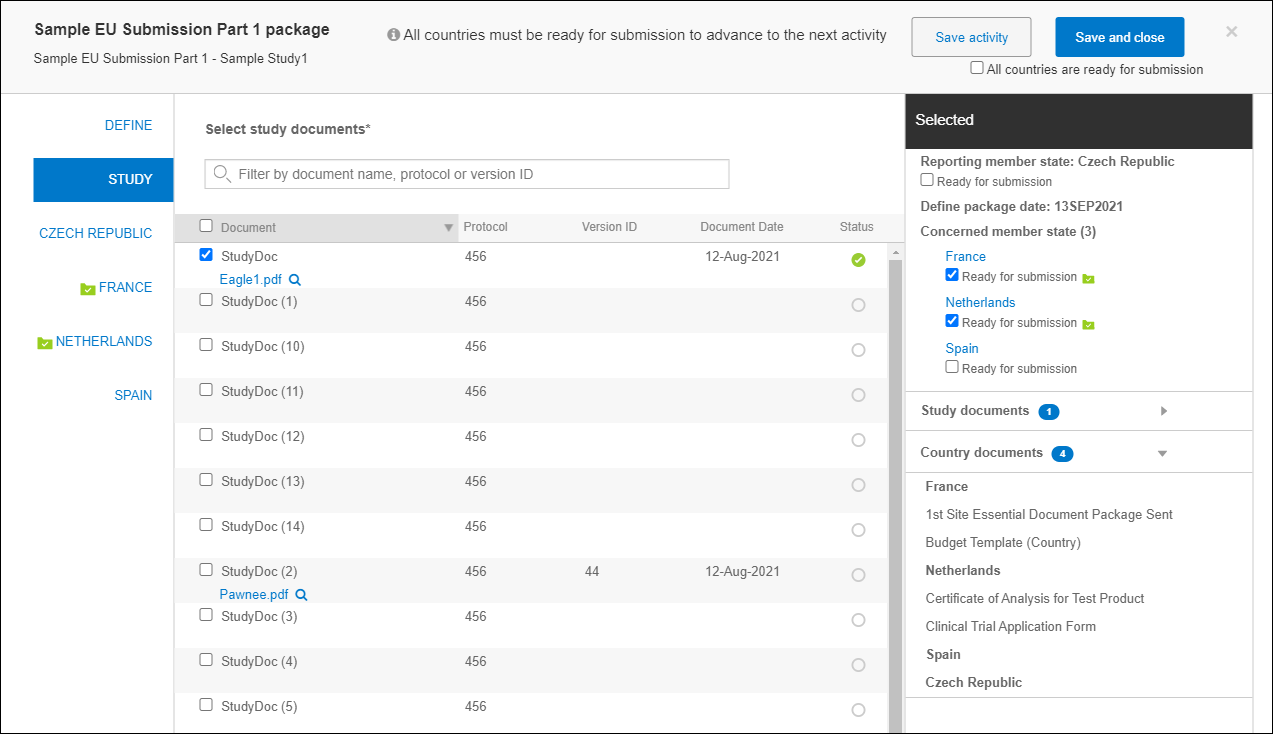
When the validation package definition has been completed as described above, you can define the EU submission Part 1 and mark states as Ready for submission, as appropriate. Like the Validation Package definition, the center panel of the Part 1 definition includes fields for:
- Reporting member state
- Define package date
- Concerned member state
For both the Reporting member state and Concerned member state(s) the value(s) automatically populate from the associated Validation package, if available. You can edit these values as necessary.
You can then select Study level and then Country level documents using the links in the far left panel or by clicking “Continue” in the modal header to advance to the next step in the definition process, study level document selection. Again, if you're working with a group of users to create the definition the modal header’s Save activity button will allow any one of the users to optionally save their progress on the definition without completing the activity. When the user enters the flow of study and country document selection, the modal header replaces the “Continue” button with a “Save and close” button.
Define package date, and chosen member state values, study documents and country documents populate the Selected panel to the far right. When appropriate, users can also check the country level “Ready for submission” check boxes in the Selected panel, or apply the ready setting to all countries in the submission package at once by checking “All countries are ready for submission” in the modal header.
When all countries are marked as ready for submission, the modal’s “Save and close” button will be replaced by a “Complete activity” button. When the user confirms the completion, the completion date for the activity is the value entered in the Define package date field.
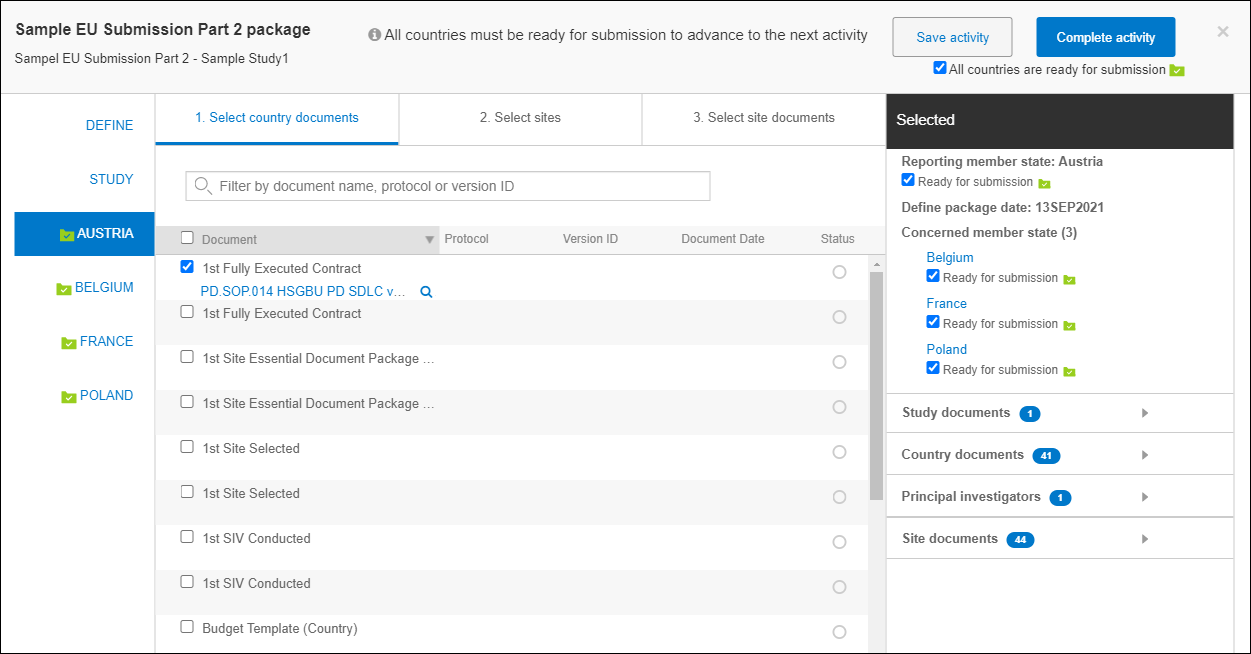
Defining an EU submission Part 2
The Part 2 definition process includes a flow and user interface functionality similar to the Part 1 definition, but after selecting Study level documents, Part 2 allows users to select country documents, sites, and site documents. Users will access these selections from the modal’s center panel, divided into three tabs.
After selection, the modal functions in the same manner as the Part 1 definition, including the ability complete the activity after all countries have been marked as ready for submission.
Submission approval confirmation steps
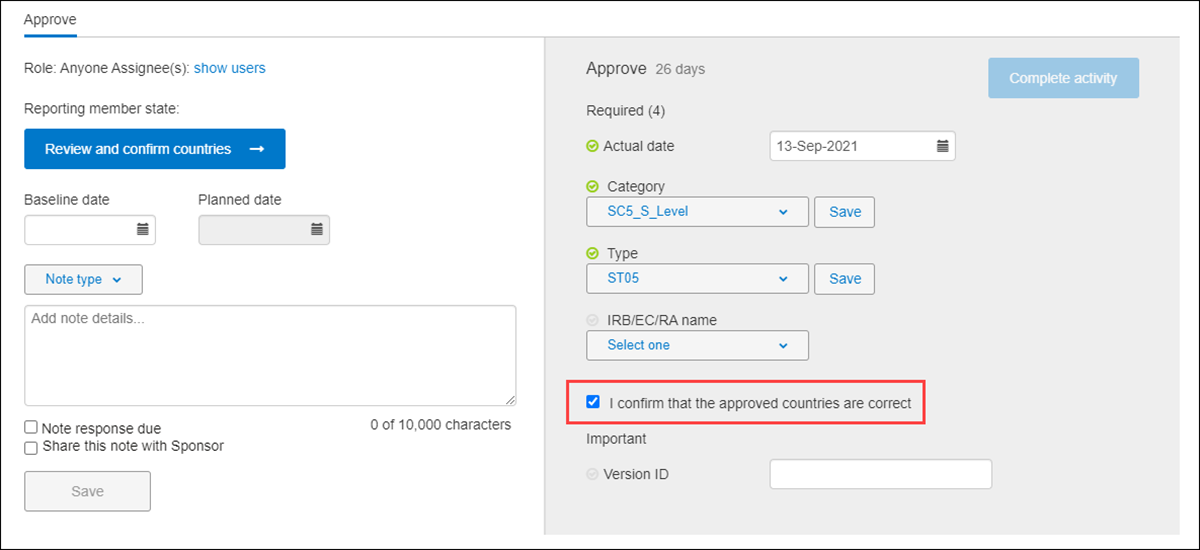
Study level submission approval activities require you to review and confirm approved countries and sites to complete the activity. Approval requirements for the available package types are as follows:
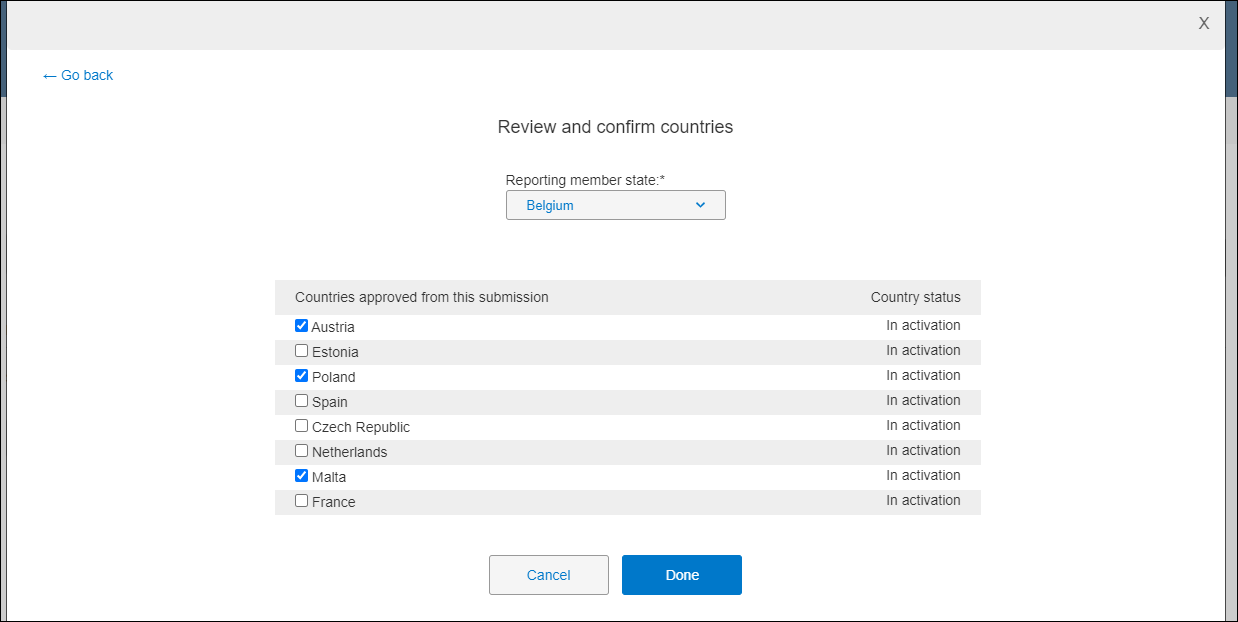
Validation package and Part 1 package – The package’s Approval Complete activity includes a Review and confirm countries button. When clicked, a modal displays a required Reporting member state field, followed by a list of all countries in the package. You'll select a value for Reporting member state, review and click the check boxes as appropriate for approved countries, and click Done to save and close the modal.
To complete the approval activity, upon return to the submission package modal, you'll check the I approve confirmed countries are correct check box in the activity completion panel. You must check the box, and complete any other required fields, to enable the Complete activity button.
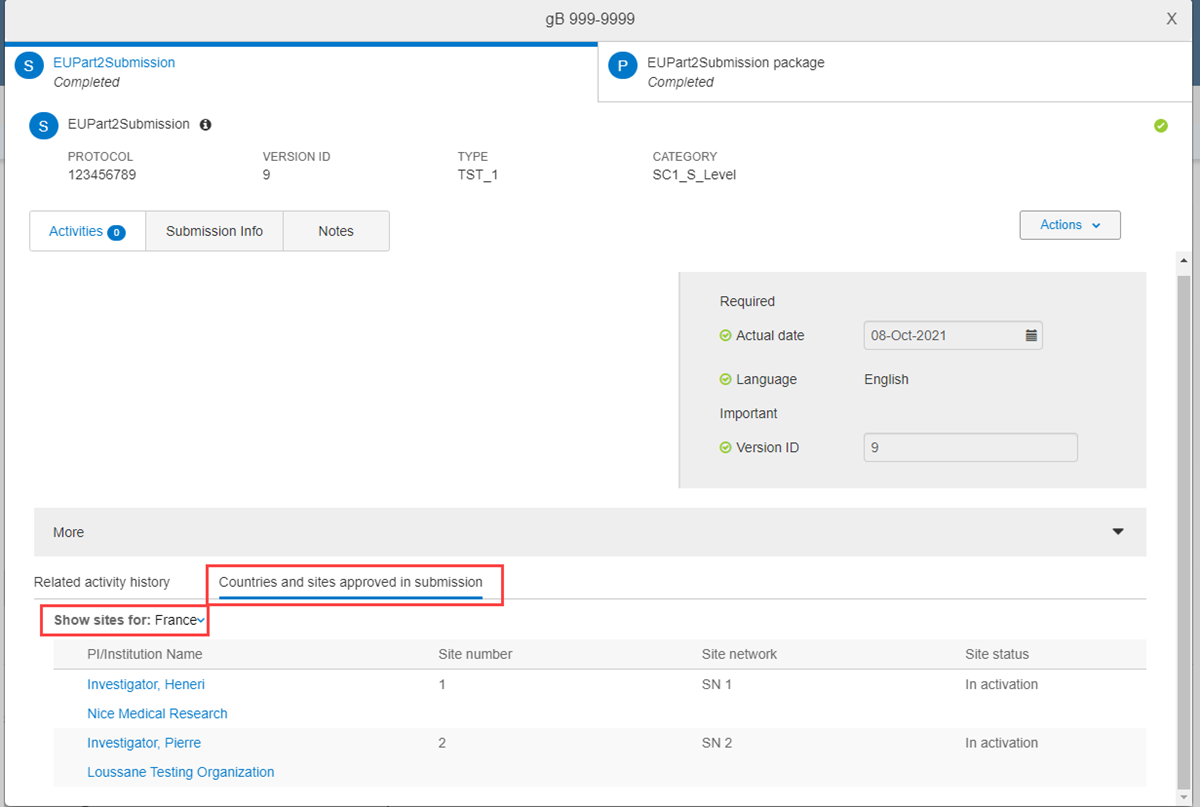
Part 2 package – This package’s Approval Complete activity is similar to the Validation package and Part 1 package design; however, the button under Activity Complete is labeled Review and confirm countries and sites. When clicked, a similar review modal displays, but in this use case, you do not have the ability to update the Reporting member state. Instead, for each reporting member state, you'll review the sites approved and check/uncheck the sites as applicable. Approvers also have the ability to remove and add a selected member state along with the state’s associated sites.
When you complete the review, the package modal displays an I confirm that approved countries and sites are correct check box in the activity completion panel. Again, you must check the box, and complete any other required fields, to enable the Complete activity button. When the check box is checked but other required fields are incomplete, the Complete activity button remains inactive.
Filter documents in the submission modal Documents in package tab
When you need to view the study, country, sites, or site documents included in a study level submission package, you can filter the package modal’s Documents in package tab to view your preferred list organized by country. The country filter defaults to the first country included in the package, by alphabetical sort. The filter list also indicates the package’s Reporting member state.
When filtered, the tab lists read-only results as follows:
- Related study documents in this package
- Related study country documents in this package
- Related study site documents in this package
- Related study sites in this package
If preferred, you can also export the package's documents using the Export to zip button.

Document export follows a defined folder structure that separates study, country, and site documents into separate folders. The folder structure is:
- Part 1 package – export file is a zip file that includes the manifest file. Within the zip file are:
- A study level folder that includes the study level files
- Separate folders for each country in the package that include the country documents for each country
- Part 2 package – export file is a zip file that includes the manifest file. Within the zip file are:
- A study level folder that includes the study level files
- Separate folders for each country in the package that include the country documents for each country. Within each country folder are:
- Separate PI name sub-folders for each site that include the documents from the sites
See the countries and sites approved in the package
Completed study level submissions associated with a Validation package, Part 1 submission package, or Part 2 submission package include a tab in the submission modal’s More section that displays country and site information as follows:
- Validation and Part 1 submissions – include a “Countries approved in submission” tab that displays the approved countries as links that open the country details page in a new browser tab. The list of approved countries also indicates which country is the Reporting member state.
- Part 2 – submissions include a “Countries and sites approved in submission” tab that displays a list of approved sites filtered by approved country. Approved sites listed in the PI/Investigator column are links that open the site details page in a new browser tab. The "Show sites for" country filter indicates which country is the Reporting member state.
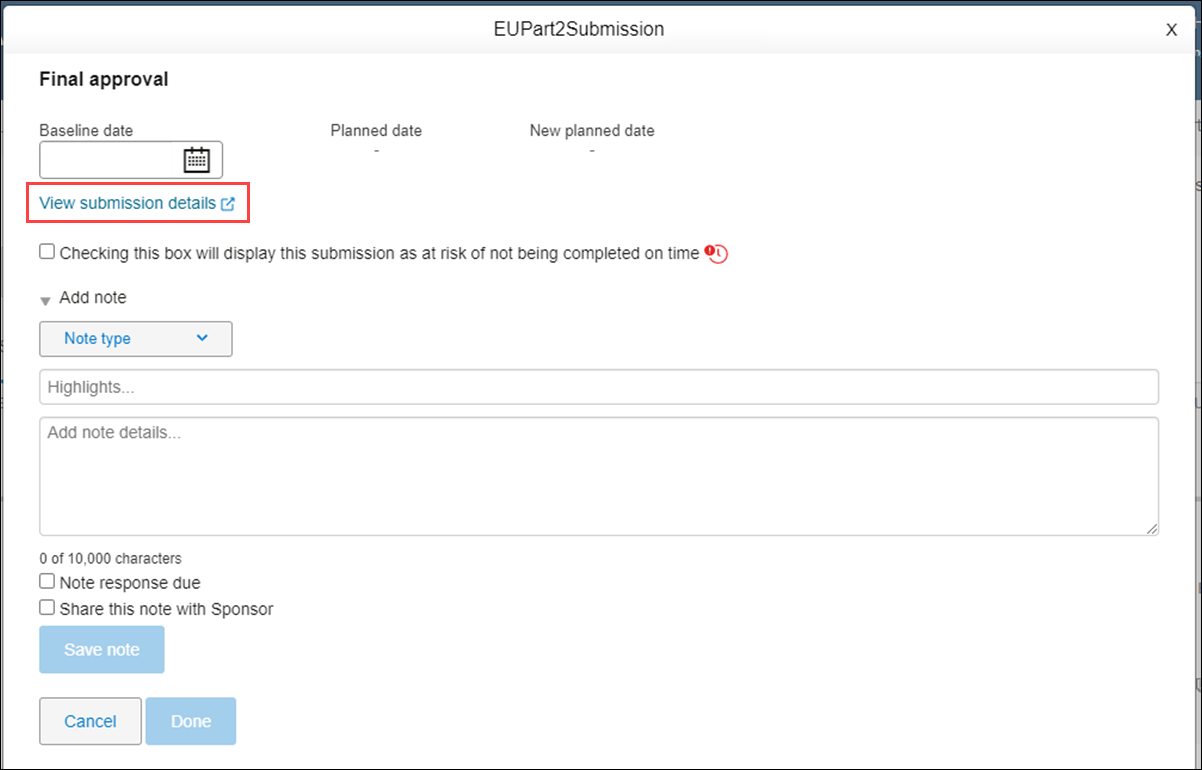
View submission details
At all levels, submission milestone modals include a View submission details link. The link displays just below the Baseline date field, and when clicked, the related submission modal opens in a new tab where you can see the countries, sites, and documents included in the submission.
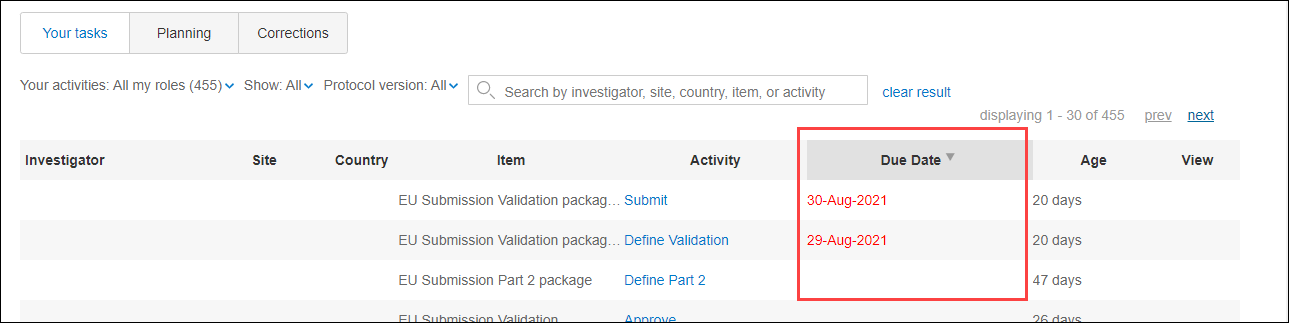
Activity level due dates and activity sorting
Regulations related to submission to the EU portal enforce specific timelines, and Oracle Site Activate supports activity level due dates. Note that this functionality is not limited to EU submission activities; activity due dates can be configured for any activity. For any configured activity, the Due date field displays in the item modal’s Activity completion panel, in the “Important” section.
Sort logic considers activity level due dates as well. The “Most past due” sort logic uses the activity due date as the primary sort, followed by the previously existing sort logic. For example, Study work – Your tasks uses activity due date as the primary sort order (activities with due dates that are most past due descending down to activities with farthest away due dates), followed by secondary sort on milestones that are most past due, and tertiary alphabetical sorting). These filters use activity level due date in sorting logic:
- Study work - Your tasks tab
- Country level - Your activities tab
- Site level - Your activities tab
- Account overview - Activities tab
Additionally, on the study Home page’s Your tasks tab, you'll find a sortable Due Date column that displays due dates for items configured for activity due date.
- Define an EU submission validation package
To create and manage study level EU submissions in Oracle Site Activate, begin by defining the validation package. - Define an EU submission Part 1
Define Part 1 after defining the EU submission validation package. In Part 1, you’ll select study and country level documents to include in the package and mark member states as ready for submission, as applicable. - Define an EU submission Part 2
Define Part 2 after defining Part 1. When defining Part 2, you’ll choose study and country level documents, sites (Principal investigators), and site level documents. - Filter documents in an EU submission package
Submission packages can include many documents. To make it easier to evaluate the list, you can filter the Documents in package tab to view documents by country. - Review and confirm countries for EU submission validation or Part 1 package
Study level submission approval activities for both the EU submission validation package and the Part 1 package require users to review and confirm approved countries. - Review and confirm countries and sites for EU submission Part 2 package
EU submission Part 2 approval activities require review and confirmation of the member state countries and sites included in the submission.