The Oracle Health Sciences Safety Suite
Suite Information
E93685-01
February 2018
The Oracle Health Sciences Safety Suite is an integrated solution for end-to-end vigilance from adverse event management to signal management, through the entire lifecycle of a medicinal product from clinical trials to post-marketing surveillance.
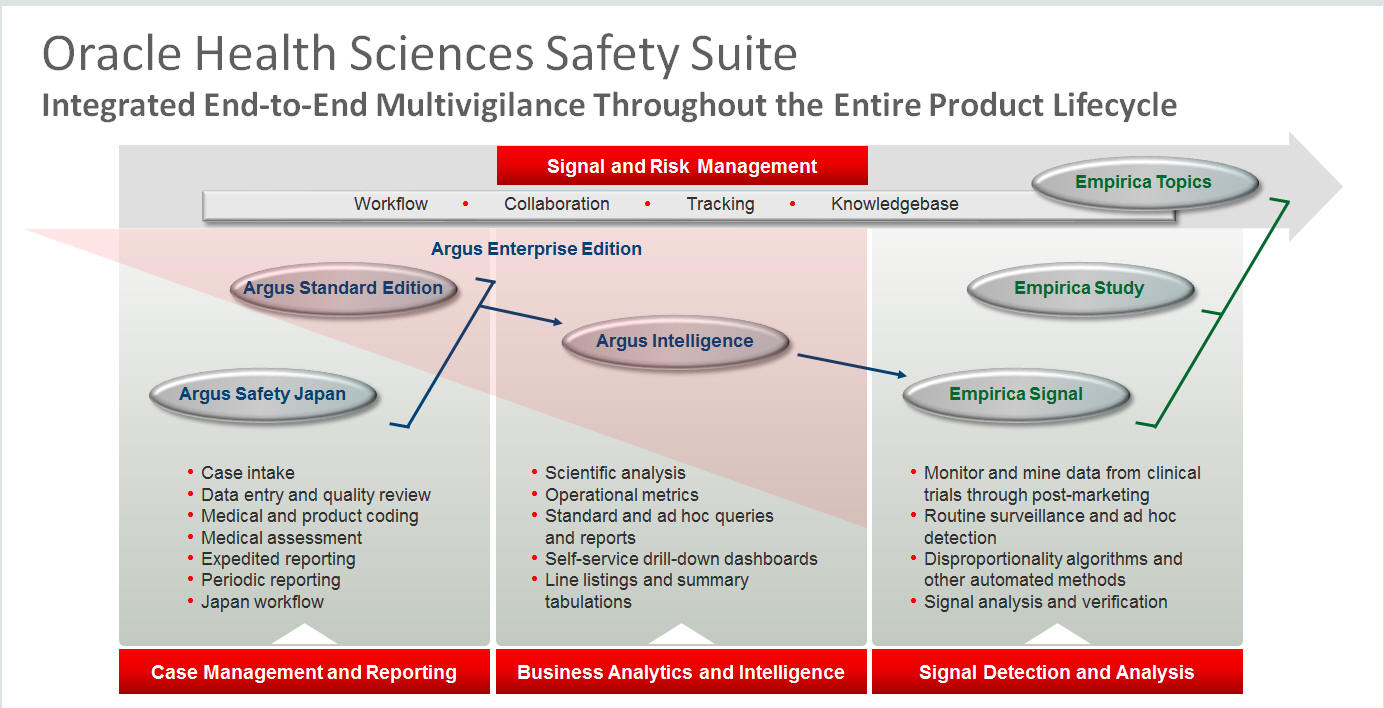
The Oracle Health Sciences Safety Suite consists of the following components:
-
Oracle Argus Standard Edition: Manage and report adverse events through a workflow including case intake, data entry, coding, quality review, medical review, expedited reporting, and periodic reporting. Modules include Oracle Argus Safety, Oracle Argus Interchange, Oracle Argus Affiliate, Oracle Argus Dossier, and Oracle Argus Unblinding.
-
Oracle Argus Enterprise Edition: In addition to managing the adverse event workflow and reporting, employ a powerful and flexible business analytics and intelligence platform for both scientific analysis and operational metrics. Modules include Oracle Argus Analytics, Oracle Argus Insight, Oracle Argus Mart, Oracle Argus Safety, Oracle Argus Interchange, Oracle Argus Affiliate, Oracle Argus Dossier, and Oracle Argus Unblinding.
-
Oracle Argus Safety Japan: Manage and report adverse events in Japan, and connect the global and local workflows using a single database.
-
Oracle Health Sciences Empirica Topics: Manage and document safety signals through a workflow including validation, prioritization, assessment, confirmation/refutation, and resulting actions.
-
Oracle Health Sciences Empirica Study: Detect and analyze safety signals in clinical trial data including adverse events, clinically significant labs, electrocardiograms, vital signs, and shifts from baseline.
-
Oracle Health Sciences Empirica Signal: Detect and analyze safety signals in post-marketing spontaneous adverse reaction data including public health authority databases and/or private inhouse databases such as Oracle Argus.
For more information on Argus Safety, visit the Oracle Health Sciences Safety suite page at:
http://www.oracle.com/goto/pharmacovigilance.html
1 Documentation Accessibility
For information about Oracle's commitment to accessibility, visit the Oracle Accessibility Program website at http://www.oracle.com/pls/topic/lookup?ctx=acc&id=docacc.
Oracle customers that have purchased support have access to electronic support through My Oracle Support. For information, visit http://www.oracle.com/pls/topic/lookup?ctx=acc&id=info or visit http://www.oracle.com/pls/topic/lookup?ctx=acc&id=trs if you are hearing impaired.
The Oracle Health Sciences Safety Suite,
E93685-01
Copyright © 2018, Oracle and/or its affiliates. All rights reserved.
This software and related documentation are provided under a license agreement containing restrictions on use and disclosure and are protected by intellectual property laws. Except as expressly permitted in your license agreement or allowed by law, you may not use, copy, reproduce, translate, broadcast, modify, license, transmit, distribute, exhibit, perform, publish, or display any part, in any form, or by any means. Reverse engineering, disassembly, or decompilation of this software, unless required by law for interoperability, is prohibited.
The information contained herein is subject to change without notice and is not warranted to be error-free. If you find any errors, please report them to us in writing.
If this is software or related documentation that is delivered to the U.S. Government or anyone licensing it on behalf of the U.S. Government, then the following notice is applicable:
U.S. GOVERNMENT END USERS: Oracle programs, including any operating system, integrated software, any programs installed on the hardware, and/or documentation, delivered to U.S. Government end users are "commercial computer software" pursuant to the applicable Federal Acquisition Regulation and agency-specific supplemental regulations. As such, use, duplication, disclosure, modification, and adaptation of the programs, including any operating system, integrated software, any programs installed on the hardware, and/or documentation, shall be subject to license terms and license restrictions applicable to the programs. No other rights are granted to the U.S. Government.
This software or hardware is developed for general use in a variety of information management applications. It is not developed or intended for use in any inherently dangerous applications, including applications that may create a risk of personal injury. If you use this software or hardware in dangerous applications, then you shall be responsible to take all appropriate fail-safe, backup, redundancy, and other measures to ensure its safe use. Oracle Corporation and its affiliates disclaim any liability for any damages caused by use of this software or hardware in dangerous applications.
Oracle and Java are registered trademarks of Oracle and/or its affiliates. Other names may be trademarks of their respective owners.
Intel and Intel Xeon are trademarks or registered trademarks of Intel Corporation. All SPARC trademarks are used under license and are trademarks or registered trademarks of SPARC International, Inc. AMD, Opteron, the AMD logo, and the AMD Opteron logo are trademarks or registered trademarks of Advanced Micro Devices. UNIX is a registered trademark of The Open Group.
This software or hardware and documentation may provide access to or information about content, products, and services from third parties. Oracle Corporation and its affiliates are not responsible for and expressly disclaim all warranties of any kind with respect to third-party content, products, and services unless otherwise set forth in an applicable agreement between you and Oracle. Oracle Corporation and its affiliates will not be responsible for any loss, costs, or damages incurred due to your access to or use of third-party content, products, or services, except as set forth in an applicable agreement between you and Oracle.