About source data
The drug safety data to be used in the Empirica Signal application might include:
- Public safety data, such as data from the Adverse Event Reporting System (AERS) or Vaccine Adverse Event Reporting System (VAERS), covered by the Freedom of Information (FOI) Act.
- Subscription data, such as data from Vigibase, the World Health Organization's Adverse Drug Reaction (ADR) database.
- Your company's own safety data.
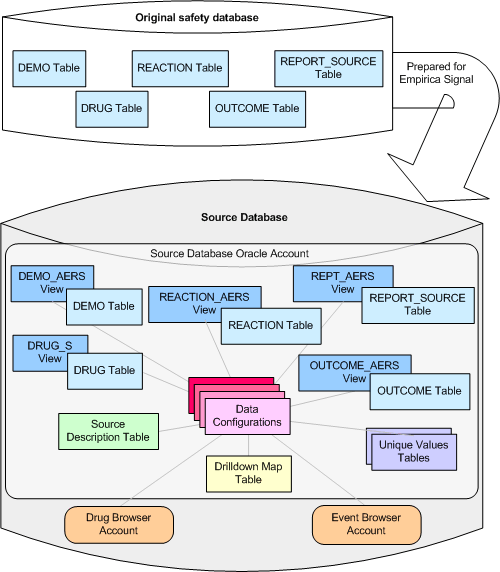
Typically, drug safety data in the original, base tables is cleaned and prepared for useful data mining. The prepared data is stored in an Oracle database and is the source data used by Empirica Signal.
Each source database resides in a separate Oracle account. In addition to the Oracle tables and views that contain safety data, the source database might include other tables that support the use of source data in the application:
- A data configuration is a group of variables that correspond to items (database columns) in the source database. There must be at least one data configuration for data mining to be performed. Typically, multiple data configurations are available. For example, there might be a data configuration for SRS+AERS data and a separate data configuration for AERS data (since 1997).
- A drilldown map table specifies which data appears when users drill down to case information.
- A source description table provides a description of the source database, such as its name and when it was loaded.
- Unique values tables enhance performance by listing unique values for variables.
If dictionaries of hierarchical data from a classification system such as the Medical Dictionary for Regulatory Activities (MedDRA) are used, each version of dictionary data is stored in a separate Oracle account. If hierarchy accounts are defined, users can browse for and select event terms or drug terms in the hierarchy.
The following illustration shows the relationship between the original safety database and the Empirica Signal source database: