In this section Hide
An all cases summary report runs on all cases in a specific data configuration. You can select report outputs from this report type in the Report Output field in a drug profile configuration. You can also add a report chart to a drug profile layout for each breakdown variable in the report definition.
To create this type of report, you create an interactive report, and then select the report type Summary of all cases. The report definition is not valid until all of the following criteria are true:
To support reports for all three subtypes on the Drugs tab, you can create a different All Cases Summary report for each of the three subtypes.
Oracle recommends that the breakdown variable and the analysis variable be from the same source table. You cannot use a continuous variable as a breakdown variable.
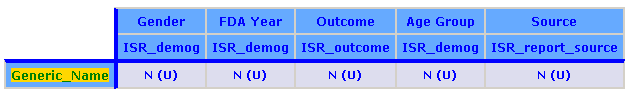
The breakdown details for each column breakdown variable are:
The content detail for the analysis variable is specified as Count (Unique).
If the report does not meet all criteria, a message appears below the report, indicating that the report is not valid. The exception to this is that the check for the drug variable subtype is not performed until you run the report.
The following example shows a report definition for an All Cases Summary report:

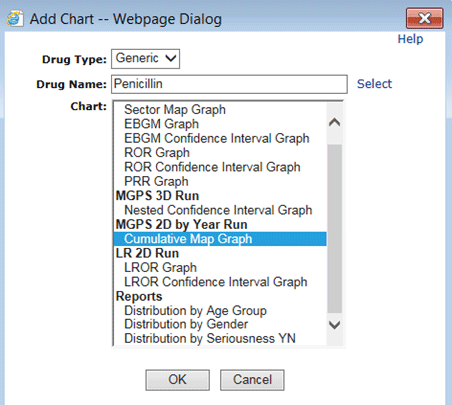
Available report charts in a drug profile include Distribution of <variable-name>, where <variable-name> is the label of the breakdown variable from the report definition. The label may differ from the variable name.
Report output for this report definition makes the following report charts available to add to a drug profile layout: