|
|
| HDR Version 7 HL7 Version 3 Messaging Conformance Specification |
| Contents Previous Next Conformance Examples |
This section contains the following topics:
5.62.1 Introduction 5.62.2 RMIM 5.62.3 Referenced CMETs 5.62.4 Links to Artifacts
The R_Drug CMET captures the Drug information that is relevant for Drug related adverse event and product reporting. This includes information about items related to the drug, and it's manufacture and sales such as the manufacturer, distributor, regulatory authority, ingredients, and generic equivalents. This CMET also captures information for therapeutic biologics, vaccines, and financial information for mass immunization programs, for example military, school, or state/local immunization programs.
This CMET is aligned with HL7 Normative Edition 2006 R_Drug CMET.
The following figure shows the R_Drug (universal) RMIM:
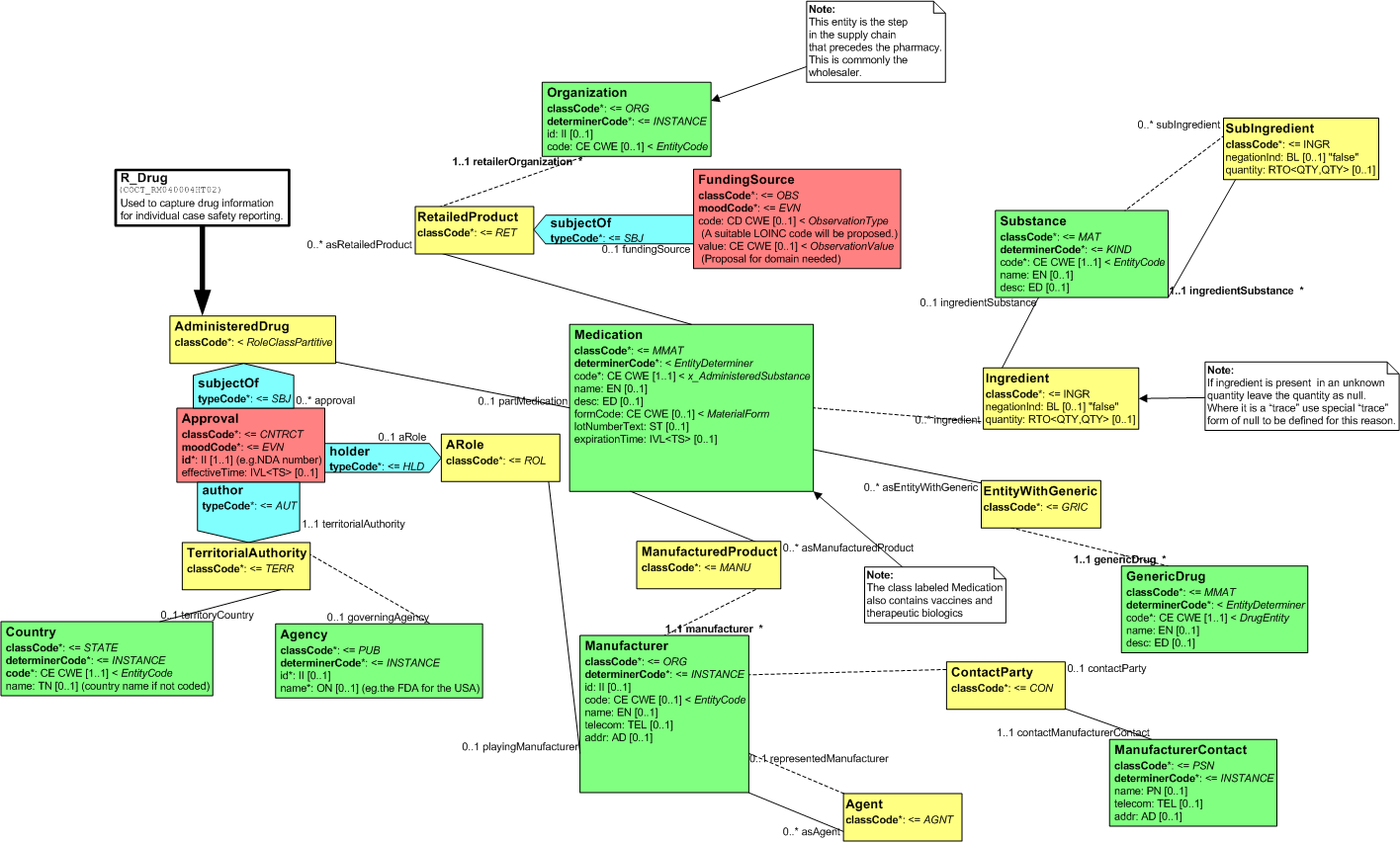
Figure: 5.62.2-1 R_Drug (universal) RMIM
| Artifacts | Link |
| Visio File | coct_rm040004ht02.vsd |
| Visio XML File | COCT_MT040004HT02.xml |
| Hierarchical Message Description (HMD) | |
| Schema | coct_mt040004ht02.xsd |
| Model Interchange Files (MIF) | coct_mt040004ht02.mif |
| Contents Previous Next Conformance Examples |
|
Copyright © 2018, Oracle. All rights reserved. |
About Oracle Contact Us Legal Notices and Terms for Use Privacy Statement |