|
|
| HDR Version 7 HL7 Version 3 Messaging Conformance Specification |
| Contents Previous Next Conformance Examples |
This section contains the following topics:
5.60.1 Introduction 5.60.2 RMIM 5.60.3 Referenced CMETs 5.60.4 Links to Artifacts
The R_Device message element type captures Device information that is relevant to device related adverse event and product reporting. This includes information about items related to the device, and it's manufacture and sales such as the manufacturer, regulatory authority, and location of the device.
A device, in this context, refers to a manufactured item that is used directly in treating and/or caring for the patient. The model captures information about devices by using one class, Device, to capture descriptive information directly related to the particular device, and another, DeviceModel, to capture information related to the kind of device.
This CMET is with HL7 Normative Edition 2006 R_Drug CMET.
The following figure shows the R_Device (universal) RMIM:
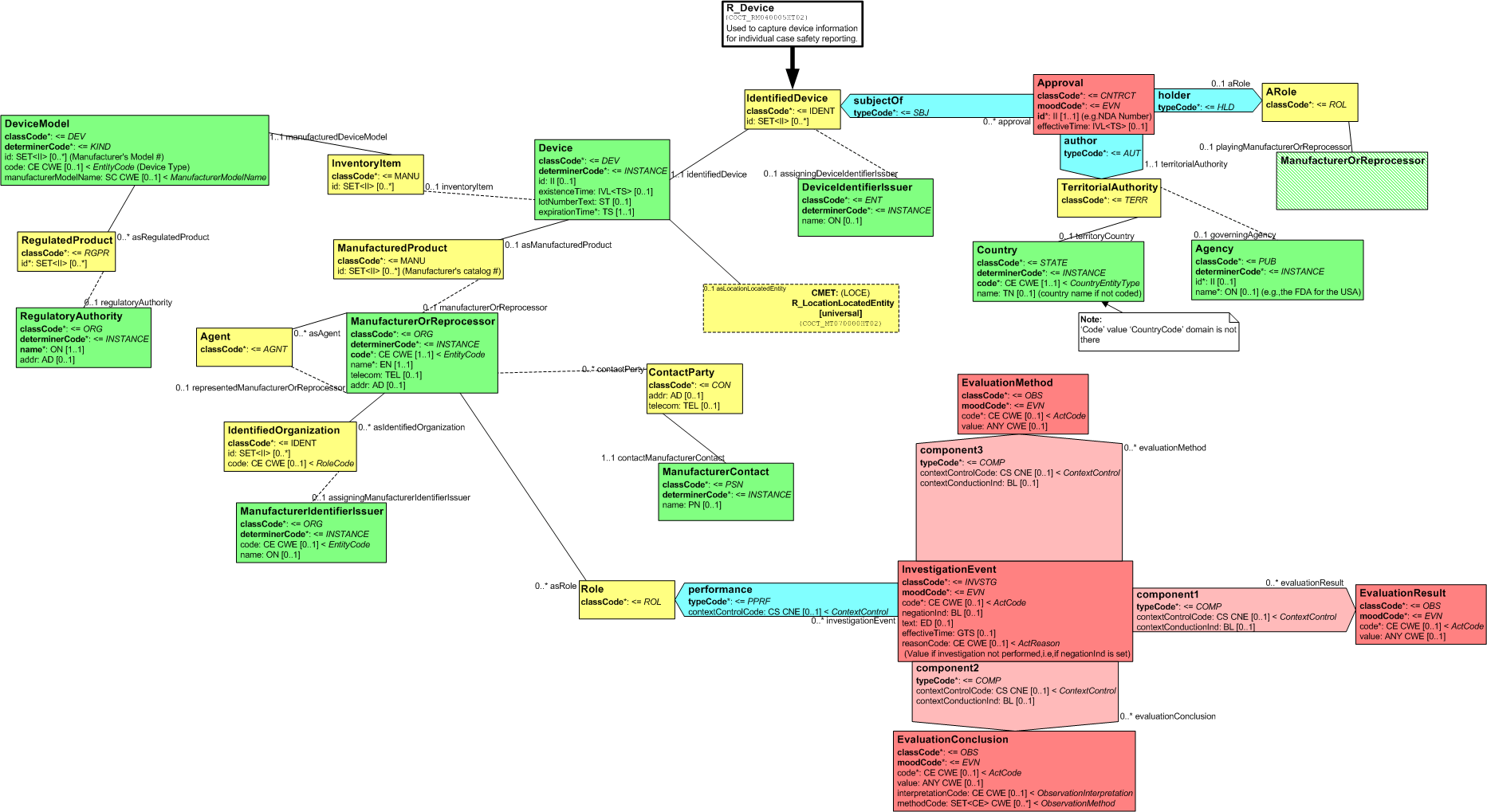
Figure: 5.60.2-1 R_Device (universal) RMIM
| Artifacts | Link |
| Visio File | coct_rm040005ht02.vsd |
| Visio XML File | COCT_MT040005HT02.xml |
| Hierarchical Message Description (HMD) | |
| Schema | coct_mt040005ht02.xsd |
| Model Interchange Files (MIF) | coct_mt040005ht02.mif |
| Contents Previous Next Conformance Examples |
|
Copyright © 2018, Oracle. All rights reserved. |
About Oracle Contact Us Legal Notices and Terms for Use Privacy Statement |