|
|
| HDR Version 7 HL7 Version 3 Messaging Conformance Specification |
| Contents Previous Next Conformance Examples |
This section contains the following topics:
4.3.1 Introduction 4.3.2 RMIM 4.3.3 State Transitions/Trigger Events 4.3.4 Interactions 4.3.5 Referenced CMETs 4.3.6 Links to Artifacts
The HDR Clinical Document Architecture (CDA) document is fully conformant to the HL7 CDA Release 2 specification. Much of the document description that follows is taken from the HL7 CDA specification.
This document is not intended as a substitute for the CDA R2 specification. Conformance to HDR CDA does not guarantee conformance to HL7 CDA R2. For details regarding the CDA R2 specification, and CDA R2 conformance information, visit HL7 Web site.
CDA is an attempt to standardize the structure of a clinical document to facilitate exchange. It is not a message specification, but rather an XML-based specification for a document containing clinical information. A CDA document may be exchanged via an HL7 version 3 message, via an HL7 version 2 message, via portable electronic media, etc. The CDA specification is independent of the transport mechanism. HDR provides a CDA document specification and a specification for an HL7 version 3 message (the Clinical Document Message) to transport the document.
A CDA document may be transmitted to HDR in the Act.text attribute of the Clinical Document Message focal act, in a MIME package. (See the section (Appendix B) on ClinicalDocumentMetadata.text for information on packaging the CDA document in the text attribute.) When received by Inbound Messaging Services, an object representing the ClinicalDocumentMetadata element will be stored, including the MIME package in the text attribute.
In addition, Inbound Messaging Services decodes the MIME package it finds in ClinicalDocumentMetadata.text and proceeds to parse the CDA document found within the MIME package. Messaging Services generates a subject ActRelationship (typeCode = SBJ) whose source is the ClinicalDocumentMetadata object and whose target is a ClinicalDocument object, the entry point of the CDA document. The RMIM objects encountered by traversing the RMIM from the ClinicalDocument object are subsequently stored in HDR.
The ClinicalDocument object is not subject to side effect configuration, that is specifying create and overlay options for an Act with classCode DOCCLIN and mood EVN in side effect configuration will not affect the processing/persistence of the ClinicalDocument object. The submitted ClinicalDocument object will always be created if an object with its id does not already exist, and will always overlay any existing object that has the id of the submitted ClinicalDocument. The ClinicalDocuments state transitions are not validated.
When Outbound Messaging Services generates a Clinical Document message, it does not validate the contents of the ClinicalDocumentMetadata.text attribute. The assumption is that the text attribute has been populated with a valid CDA document, contained within a MIME package.
A CDA document is wrapped by the
The CDA document body: the content bounded by the
A
The CDA narrative block is wrapped by the
CDA entries can nest and they can reference external objects. CDA external references always occur within the context of a CDA entry. External references refer to content that exists outside this CDA document - such as some other image, some other procedure, or some other observation (which is wrapped by the 'referredToExternalObservation element). Externally referenced material is not covered by the authentication of the document referencing it.
The following figure diagrams the CDA Document RMIM:
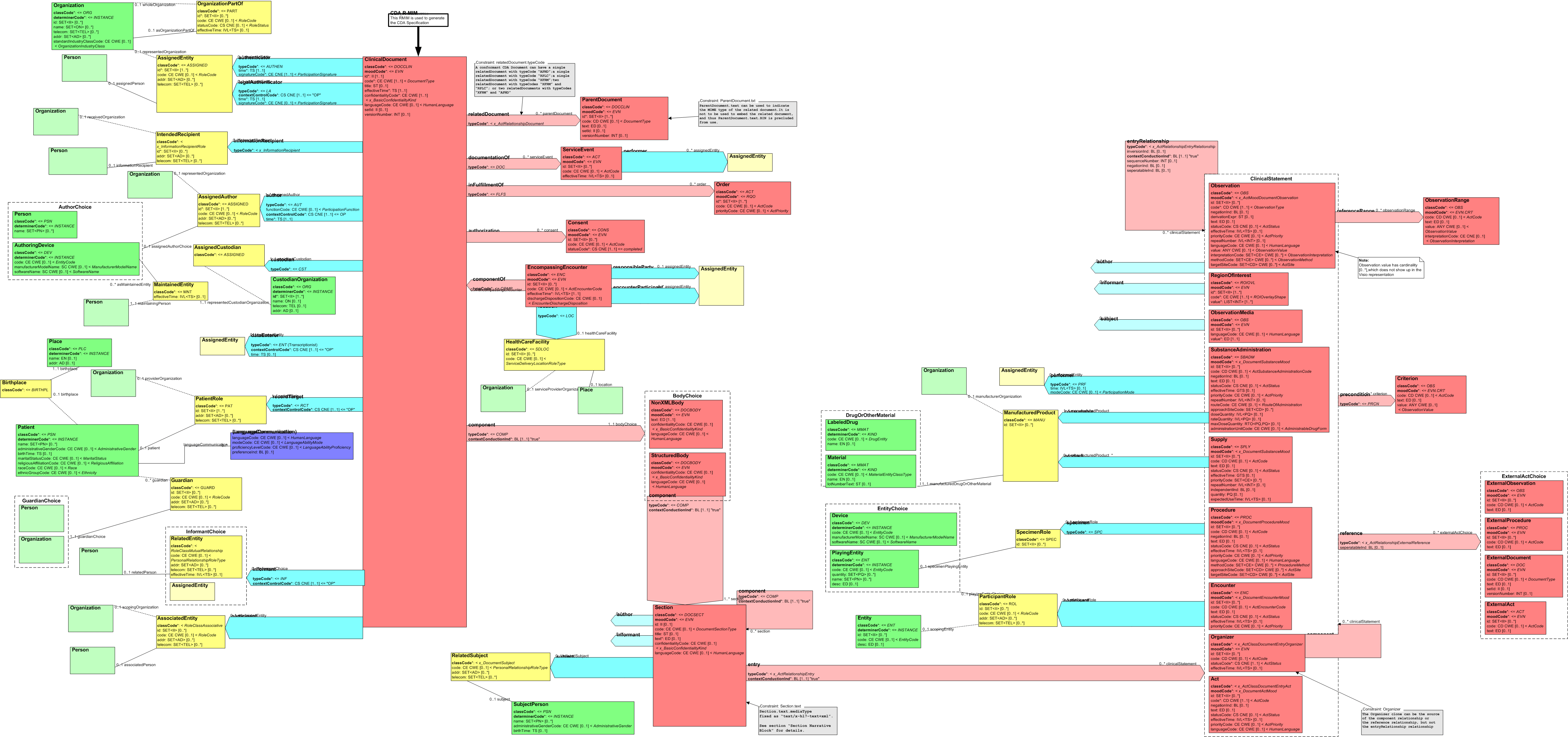
Figure: 4.3.2-1 CDA Document RMIM
| Artifacts | Link |
| Visio File | pocd_rm000040ht01.vsd |
| Visio XML File | pocd_rm000040ht01.xml |
| Hierarchical Message Description (HMD) | pocd_hd000040ht01.xls |
| Schema | pocd_mt000040ht01.xsd |
| Model Interchange Files (MIF) | pocd_mt000040ht01.mif |
| Side Effect Configuration Spreadsheet (for use in WebADI) | pocd_mt000040ht01.csv |
| Interaction Schemas | (File not included) |
| Contents Previous Next Conformance Examples |
|
Copyright © 2018, Oracle. All rights reserved. |
About Oracle Contact Us Legal Notices and Terms for Use Privacy Statement |