|
|
| HDR Version 7 HL7 Version 3 Messaging Conformance Specification |
| Contents Previous Next Conformance Examples |
This section contains the following topics:
4.5.1 Introduction 4.5.2 RMIM 4.5.3 State Transitions/Trigger Events 4.5.4 Interactions 4.5.5 Referenced CMETs 4.5.6 Links to Artifacts
The Clinical Trial Laboratory Observation Periodic Report RMIM is used for communicating clinical trials laboratory result information. The model represents the administrative aspects of a clinical trial (protocols, trials, sites, investigators, subjects and study events), and the information requirements for representing the laboratory observations generated during the conduct of a clinical trial. It also represents the set of actions that define an experiment to assess the effectiveness and/or safety of a biopharmaceutical product (food, drug, device and so on). In the event mood, this designates the aggregate Act of applying the actions to one or more subjects.
For HDR conformance, it is expected that the following values have been defaulted prior to the receipt of the message by the Inbound Message Processor. This must be done by either the source system or the message transport mechanism (Interface Engine).
| Attribute | Default |
| component/trialAtSite/component/investigatorAtSite/component/subjectAssignment/ component/studyEvent/component/accession/component/baseSpecimenDefinition/subject/ baseSpecimen/productOf/specimenCollection/pertinentInformation2/pertinentAgeAtVisit/code | 000817/HDR Supplemental |
| component/trialAtSite/component/investigatorAtSite/component/subjectAssignment/ component/studyEvent/component/accession/component/baseSpecimenDefinition/subject/ baseSpecimen/productOf/specimenCollection/pertinentInformation1/pertinentFastingStatus/code | 001818/HDR Supplemental |
The following figure diagrams the Clinical Trial Laboratory Observation Periodic Report RMIM:
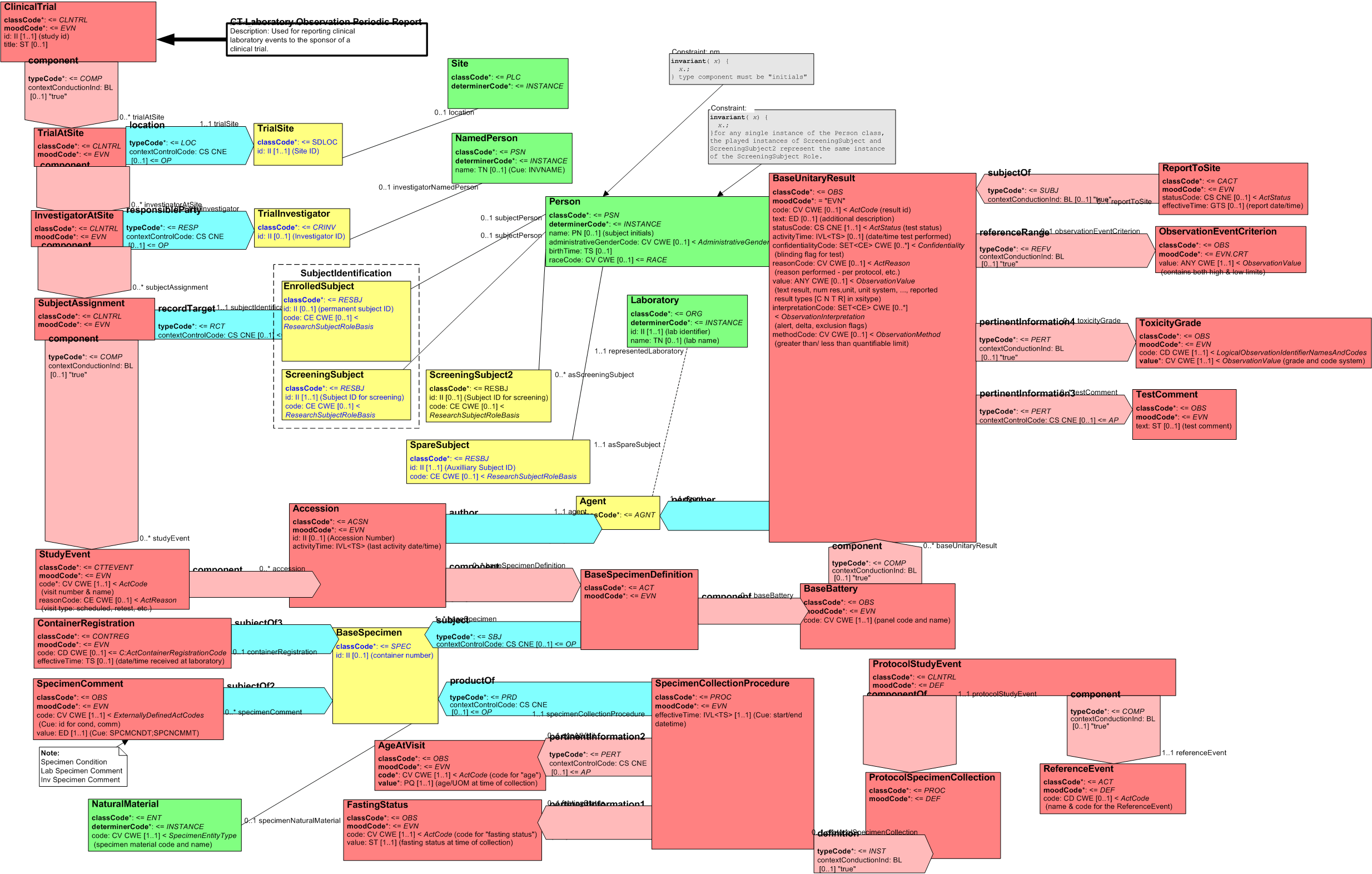
Figure: 4.5.2-1 Clinical Trial Laboratory Observation Periodic Report RMIM
The list of trigger events follows:
Table 4.5.3-1 Clinical Trial Laboratory Observation Periodic Report - Trigger Events| Begin State | End State | Trigger ID | Trigger Event |
| PORT_TE010001 | Clinical Trial Agreed Transmission Criterion Attained |
The list of Interactions follows:
Table 4.5.4-1 Clinical Trial Laboratory Observation Periodic Report - Interactions| Interaction ID | Trigger Event | Transmission Wrapper | Control Act Wrapper |
| PORT_IN010001 | PORT_TE010001 | MCCI_MT000100HT04 | MCAI_MT700200HT01 |
| Artifacts | Link |
| Visio File | port_rm030001hl02.vsd |
| Visio XML File | port_rm030001hl02.xml |
| Hierarchical Message Description (HMD) | port_hd030001hl02.xls |
| Schema | port_mt030001hl02.xsd |
| Model Interchange Files (MIF) | port_mt030001hl02.mif |
| Side Effect Configuration Spreadsheet (for use in WebADI) | port_mt030001hl02.csv |
| Interaction Schemas | port_in010001.xsd |
| Contents Previous Next Conformance Examples |
|
Copyright © 2018, Oracle. All rights reserved. |
About Oracle Contact Us Legal Notices and Terms for Use Privacy Statement |