| Oracle® Clinical Remote Data Capture Classic Data Entry User's Guide Release 4.6.2 Part Number E18824-01 |
|
|
View PDF |
| Oracle® Clinical Remote Data Capture Classic Data Entry User's Guide Release 4.6.2 Part Number E18824-01 |
|
|
View PDF |
This section describes tasks you perform during initial data entry. Just as there is a progression in data entry status that is linked to the workflow, the tasks that are considered part of initial data entry also represent steps in a progression.
The tasks that can be part of initial data entry are:
Certain tasks, such as "Initiate Data Entry", are limited by definition to initial data entry, while others, such as "Initiate a Section Discrepancy" and "Initiate an Investigator Comment" can occur at any point in the data entry workflow, up until the CRF is locked.
Note:
The procedures in this section reference various GUI components and assume knowledge of basic functionality. If you have questions about components used in the tasks, refer to the Part III, "RDC Classic GUI Reference" sections.In order to open a CRF, your user name must be assigned the browse privilege, at a minimum (browse batch privilege for batch loaded data). In order to record or update patient data, your user name must be assigned the update privilege.
To open a CRF:
Locate the correct CRF in the RDC Classic Spreadsheet.
Refer to "RDC Classic Spreadsheet" for information on locating a specific CRF.
Click the CRF cell. The system opens the CRF in the Data Entry window.
Note:
If the system does not open the Data Entry window when you click the CRF cell, ensure that the "Action When Clicking on a Cell" setting is selected. (For details about this setting, refer to "Preferences Window".)This is the first step in the data entry process. In order to perform data entry, your user name must be assigned the update privilege.
Open the CRF in the Data Entry window and place focus in the CRF header area.
Complete all of the required CRF header information fields. If a CRF section header area is present, place focus in it and complete all required fields there.
Place focus in the first response field in the Question area and begin to record data in the appropriate fields. Complete all fields in the section.
If there is another CRF section:
Place focus in the CRF section header and complete all required fields.
Place focus in the first response field in the Question area and begin to record data.
Repeat these steps for all subsequent sections in the CRF.
When all CRF information and response fields are complete, you can:
Save pending changes and proceed to the next CRF.
Save pending changes and close the CRF.
When all required CRF header data is collected and you save the CRF, RDC Classic assigns it the data entry status created.
A CRF can have one or more fields into which you insert data.
A standard text field can have up to 200 characters. Most fields in a CRF are standard text fields.
An extended text field can have up to 10,000 characters.
Any field in the CRF that has an ATTACHMENT prefix represents an extended text field that supports 10,000 characters instead of the normal maximum of 200 characters.
As shown in Figure 8-1, RDC Classic uses the following prefix to indicate that a field is an extended text field:
ATTACHMENT V <X.Y>:
where X is the version number and Y is the sub-version number. The system increments these numbers each time the data in an extended text field is saved.
After the ATTACHMENT prefix, RDC Classic displays the beginning of the extended text. For example:
ATTACHMENT V<1.1>: A 48-year-old woman presented
Note:
In RDC Classic, you can view only the beginning of the data in an extended text field. You cannot view all the data in the field. In addition, you cannot enter or update data in the field. You can enter and modify data in an extended text field only in Oracle Clinical Remote Data Capture Onsite (RDC Onsite).You use the Investigator Comment Window to add a new comment. In order to complete this task, your user name must be assigned the update privilege.
To insert an investigator comment in a CRF:
In the Question area, place focus in the relevant response field.
From the Insert menu, choose Investigator Comment. The Investigator Comment Window opens.
Type the comment in the text box.
When you are ready to close the Investigator Comment window, click OK. The system saves the investigator comment.
When you save the comment, focus returns to the response field. The presence of an investigator comment associated with the data field is signified by:
The yellow background color of the field
The presence of <Inv> appended to the contents of the Data Entry window title bar
As you record data in response fields and navigate to other fields, RDC Classic evaluates the value you record. When you record a value in a response field that does not meet certain criteria defined for the question, the system displays the Validation Failure Window, which indicates the presence of a discrepancy. When the validation error window displays while you are recording data, there are three routes you can take:
Return to the response field and correct the value
Acknowledge the discrepancy and proceed with data entry
Acknowledge and take action on the discrepancy
To process the validation error:
Ensure the response value that raised the discrepancy matches the source data.
If the value is recorded correctly, skip to Step 3.
If the value is not recorded correctly, click the Cancel button. The system closes the Validation Failure window and returns focus to the response field, where you can correct the response value.
If the system again displays the Validation Failure window, the new value is also discrepant; proceed to Step 2.
If the value in the response field matches the source data, you can either:
Acknowledge the discrepancy and proceed with data entry
Acknowledge and route the discrepancy
Acknowledge and resolve the discrepancy
To acknowledge a discrepancy:
In the Validation Failure window, review the Error Message text box.
If you want to augment the error message, type a text message in the Comments text box.
If you want to take action on the discrepancy, that is, either route or resolve it, proceed to Step 4. If you want to proceed with data entry, skip to Step 6.
Select an action in the Action drop-down list box:
If you select a routing action, such as "Send to Site", skip to Step 6
If you select a resolution reason, such as "Close - Resolved", the system displays the Resolution Reason window, proceed to Step 5.
In the Resolution Reason window,
Select a resolution reason from the Resolution drop-down list
Type an optional comment in the Comment text box that you want to save with the discrepancy
Click Save; the system closes the window and returns focus to the Validation Failure Window.
Click the OK button. The system closes the Validation Error window.
If the CRF is not in entry complete status, the system returns focus to the Data Entry window and you can continue with initial data entry.
If the CRF is in entry complete status, the system displays the Audit Information Window, in which you must the change reason.
When you complete the audit information window, the system saves the discrepancy record and the change reason, and it reverts focus to the response field.
The discrepancy actions that are available are based on your user role and the setup done by the system administrator.
Correcting the value in the response field – When the system presents the Validation Failure window, the Message section displays the system-generated reason that the data value is discrepant. The response value is included in the reason. This allows you to compare the value that you typed in the response field to the source data value. If the two values do not match, use this procedure to navigate back to the response field and correct the value you typed.
You can mark a CRF blank in order to signify that it was not overlooked during data entry and that it was intentionally left blank. When a CRF is marked blank, you can, at a later time, clear the blank flag and proceed with normal data entry.
If data has been recorded in the CRF when you mark it blank, the system deletes all response values in the CRF.
To mark a CRF blank:
Open the CRF.
Complete all required CRF header fields.
If necessary, complete any optional CRF header fields.
Complete all required CRF section header fields, if applicable.
Click the blank flag check box. The system displays the Blank CRF Confirmation Window with the message, "Mark this CRF blank and Save?"
In the confirmation window, click OK to mark the CRF blank and save pending changes.
When you complete the task, the Blank check box is selected and the CRF question area is inaccessible for data entry.
This task, which is applicable to multi-section CRFs, allows you complete initial data entry for one or more CRF sections and mark one or more other CRF sections blank.
RDC Classic allows you to mark individual sections of a CRF blank when the CRF blank flag is not selected. This is useful when all of the data to be collected in the CRF is not available. In this way, you can record the data on hand, mark those sections for which data is not yet available blank, and save the CRF.
To mark one or more sections blank in a multi-section CRF:
In the RDC Classic Spreadsheet, locate and open the CRF you want to create.
Ensure that all required CRF and CRF section header fields are complete.
Place focus in the CRF section you want to mark blank.
Select the blank flag check box.
If all other sections in the CRF are marked blank, RDC Classic displays a window that requires you to confirm your choice and save pending changes.
If other sections are not blank, the system displays a text message in the current section, "This section has been marked as blank".
If another CRF section must be marked blank, navigate to it and repeat this procedure.
Note that when all other sections in the CRF are marked blank and you mark the last CRF section blank, RDC Classic automatically marks the entire CRF blank.
Under certain circumstances, you can add a row to a set of repeating question rows. This facilitates collection of additional data in a question group.
To insert a repeating question group row:
Place focus in the row above the one you want to delete.
From the CRF menu, choose Insert Row menu command.
The system adds the row, if necessary, by moving the rows below down one row.
The conditions that prevent you from inserting a row in the question group are related to how the question is defined in Oracle Clinical. The question group may be protected against the insertion and/or deletion of additional rows, or it may contain the maximum number rows specified in the definition. In these cases, the system displays a message window that informs you that a row cannot be added to the question group.
Note that if your user name is assigned privileged update, the system does not enforce these restrictions.
A user-generated discrepancy can provide additional information about a response field. In contrast to an investigator comment, the discrepancy can be routed to another user group and it is reflected in the color-coding of the CRF icon that is displayed in the RDC Classic Spreadsheet.
This section describes the procedure to add a discrepancy to a response field during initial data entry. One user-generated discrepancy can be associated with each response field in the question area of a CRF section.
To initiate a discrepancy for a response field:
Place focus in the response field of the question with which you want to initiate a discrepancy.
Select Insert, then Discrepancy from the option list. The system opens the Comment for Question Window. Ensure that the title bar refers to the correct question label.
In the discrepancy window:
Ensure that the Comment Reason is correct. If it is not, use the LOV button to select an alternative.
Type explanatory text in the Message pane. If necessary, click the Editor button to open the Text Editor.
You can use a section discrepancy, which is associated with the CRF section that is active when you insert it, to log comments about the questions and/or response data in that section, to facilitate communication between workers, or to use the internal discrepancy feature of RDC Classic.
For single-section CRFs, the procedure is straightforward, the section is active as long as the CRF is open. For multi-section CRFs, where each section is represented by a tab within the Data Entry window, the section that is active is determined by which tab is currently selected.
To insert a section discrepancy:
Ensure that the correct CRF is open in the Data Entry window and the section with which you want to associate the discrepancy is active.
Select Data Entry, then Discrepancy from the option list, which opens the Discrepancy for CRF Section Window. Refer to Figure 8-2.
Figure 8-2 Discrepancy for CRF Page Window
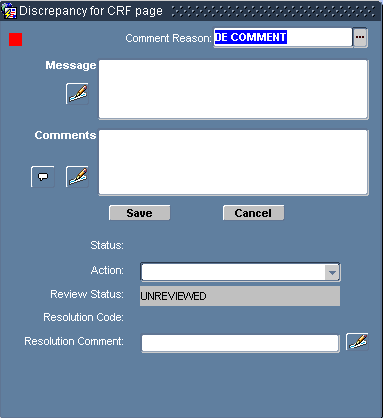
In the Discrepancy for CRF page window:
Ensure that the appropriate Comment Reason is chosen for the Comment Reason field.
In the Message text box, type the message that you want to associate with the discrepancy.
Use the Comments text box to type an additional comment that you want to associate with the discrepancy.
Click the Save button. The system saves the discrepancy, closes the window, and, if you selected an action, takes the action you specified.
The Message text you type is displayed in the "Text" field of the List of Discrepancies Tab and as the descriptive title of the Individual Discrepancy Tab in the Discrepancy.
The Comments text you type is displayed in the Comments fields of both the List of Discrepancies Tab and the Individual Discrepancy Tab in the Discrepancy.
You have the option to immediately route or resolve the discrepancy. Use the Action drop-down list box to select an action, if necessary.
When you close a CRF, there are several factors that determine the steps you must perform. The factors are:
The entry status of the CRF
Whether or not values for response fields were previously entered and saved
The presence of required fields in the CRF header
The presence of mandatory fields in the question area
The presence or absence of pending changes
In practice, these factors tend to be inter-related. For example, if the CRF is assigned an data entry status of Created, but not all required header fields are collected, RDC Classic does not allow you to close the CRF.
Essentially, RDC Classic does not assign a data entry status to a CRF until all required header fields, both CRF and CRF section header fields, are collected. If you attempt to close a CRF with required header fields uncollected, the system places focus in the first uncollected required header field, as a prompt to you.
If there are pending changes, a confirmation message window is displayed that allows to save changes and close the CRF, remove pending changes and close the CRF, or remove pending changes and display the CRF with previously saved data only.
In each of these cases, if there are no changes pending, the selected action proceeds without an opportunity for you to cancel it.
There are four methods you can use to close a CRF:
Click the X button in the top right-hand corner of the Data Entry window.
Click a Page Grouping tab other then the one currently in focus.
Click another CRF cell.
Exit the application.
In each case, RDC Classic closes the current CRF if:
There is not an uncollected required header field.
There are not pending changes.