| Agile Product Lifecycle Management Getting Started with Agile Management for Pharmaceuticals Release 9.3.3 E39289-01 |
|
 Previous |
 Next |
| Agile Product Lifecycle Management Getting Started with Agile Management for Pharmaceuticals Release 9.3.3 E39289-01 |
|
 Previous |
 Next |
Welcome to the Getting Started with Recipe & Material Workspace. This manual describes the main features and capabilities of Agile Recipe & Material Workspace (RMW) within the Agile PLM application suite. It describes the concepts, actions, and navigational flows that are used in the RMW solution, and should be considered a starting point for new users of the application.
This manual primarily addresses RMW users who perform various roles within the pharmaceutical drug development process, from early experiments to pilot labs through scale-up. Once the process and recipe is proven, the recipe can be transferred to large scale commercial production. Typical users are Process Scientists, Analysts, Material Managers, Equipment Managers, and others involved in the drug manufacturing processes.
Before you start working in RMW, read this manual to understand the following:
Concepts and components of RMW.
Initial actions that you need to perform to begin working with the application, and basic navigation. See also: "Navigating in Agile RMW."
Task and workflow management. See also: "Workflow Routings."
Searching for business objects in RMW. See also: "Working with Searches."
Reporting capabilities. See also: "Working with RMW Reports."
Standards you can create or associate with material or equipment. Standards can be reused and referenced in a recipe.
Environmental aspects such as environmental conditions for container storage, or room temperature.
The complete list of RMW manuals is provided here for the benefit of users and administrators of the RMW solution.
Getting Started with to Agile Recipe Management for Pharmaceuticals - describes common concepts, basic navigation, searches and workflows. Also covers how to work with reports, standards, and environmental conditions.
Agile Recipe Management for Pharmaceuticals Administrator Guide - describes all administration and configuration information including Agile PLM integration requirements.
Agile Recipe Management for Pharmaceuticals Process Management Guide - describes the features of the Process module, covering the creation and execution of projects and campaigns, control recipes, and work requests.
Agile Recipe Management for Pharmaceuticals Recipe Management Guide - describes the features of the Recipe module, covering the authoring and management of recipes and recipe templates.
Agile Recipe Management for Pharmaceuticals Material Management Guide - describes the features of the Materials module, covering how to work with material requests, inventory, and allocation. Also covers how to manage analytical activities.
Agile Recipe Management for Pharmaceuticals Equipment Management Guide - describes the features of the Equipment module, covering equipment qualification, loan, lease, and reservation.
Agile Recipe Management for Pharmaceuticals Import/Export Guide - describes how to export and import RMW business and administrator objects from a source system to a target system.
RMW is accessed only through the Agile PLM user interface. Refer to Getting Started with Agile PLM along with the Agile PLM Administrator Guide for a thorough understanding of PLM processes. The complete set of Agile PLM documentation, including RMW documentation, is available on the Oracle Technology Network (OTN) Web site http://www.oracle.com/technetwork/documentation/agile-085940.html.
RMW is a Web-based solution provided with the Agile PLM suite to cater to the needs of the pharmaceutical development industry. It is made up of several dimensions such as Recipe (Instructions), Equipment, Material, Analytical (Test and Assays), Environment, Standards and People. These dimensions enable drug manufacturers to conduct the preparation, execution and analysis necessary during the scale-up life cycle of a substance across multiple pilot plants, located in disparate geographic locations. It also helps scale up material production in a systematic and reproducible manner.
RMW provides an extensive view of all activities involved in drug manufacturing. It allows aggregation of information over the lifecycle of a drug product and offers the following benefits:
Information management of Product Development Lifecycle for drug substance and drug product.
Planning, execution, and analysis of campaigns, process definitions, and work requests.
Integrated view of processes, materials, equipment, environment, people, and standards data.
Conformance to business, science, and compliance metrics.
Easy access to data in order to enable tech transfers, reviews, submissions, collaborations, and distributed operations.
Consolidated views of lots, campaigns, and so on.
Product development record, creation of transaction traces (or material chain of custody,) material genealogy archives, and recipe genealogy archives.
Secure role based access for all.
Role management that defines various roles for access to the system.
Efficient streamlining of work using alert management.
Compliance with Code of Federal Regulations Part 11 through audit trials.
Custom workflows support.
Metamodeling ability to define and build a classification hierarchy for the custom object sets for Material, Equipment, and Standards.
Output Material made in the Process or Work Request displays correctly.
Output Material (OM) quantities made in a Process Step or Work Request now displays and ensures that:
Process Step (PS) specifies the number of Control Recipes (CR), as well as planned Output Material quantity for the PS itself in the Output tab;
Number of CRs specified within the PS is now used to populate the planned Output Material quantity within each Control Recipe of this PS;
Once the Work Request makes the Output Material, the actual Output Material quantities are correctly displayed in the Work Request and its Control Recipe and associated Process Step; and,
The view of Campaign Summary at the Project level reflects the Output Material quantities consistently.
To add Materials and Equipment directly to a Recipe, users previously had to create variables, and then resolve the variables. Now:
Users can add Materials and Equipment directly onto the BOM and BOE.
User must indicate which Recipe Action is using that resource.
Similarly, while editing a Recipe, Process Step, or Work Request, users can edit a Recipe Action instance and add Materials, Equipment, or Standards directly without creating variables.
The view for a Lot supports Stability Studies:
Study dates;
Study environmental conditions; and,
Results of sample testing.
Containers that you consume in a Work Request are displayed, completing the reverse trace (backward genealogy) of how a Material was made.
Parameters values that you enter show up while navigating in RMW with other details (For example, Materials consumed, Equipment used) in a Work Request that is closed out. For more details, see the Agile Recipe Management for Pharmaceuticals Process Management Guide.
RMW covers the entire lifecycle of a drug product from the lab to its commercial launch. The following diagram shows the major components of RMW.
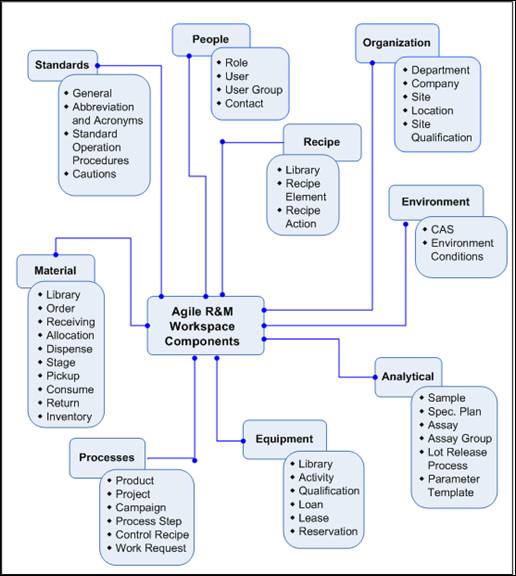
Description of the illustration ch1_01.jpg
The capabilities of each component are described in the following topic. For detailed information and instructions on how to use these components effectively for your business process, see the relevant user guide of each component.
Here is a brief description of each component appearing in RMW.
A Recipe contains the minimum set of information about the developmental requirements of a specific product. Recipes generally include instructions about gathering and combining raw material, the type of equipment to be used, and processes to be followed to create the target product.
For details, see Agile Recipe Management for Pharmaceuticals Recipe Management Guide.
People Management enables you to set up users, roles, user groups, and contacts. This is done by the administrator.
Material Management enables you to classify and organize material used in the development process and track various inventory transactions. The system is similar to an ERP system, tracking resources in a fast-paced, changing development environment. The ultimate goal is to create a recipe which is well proven in a development area, before transferring to commercial operations. For details, see Agile Recipe Management for Pharmaceuticals Material Management Guide. 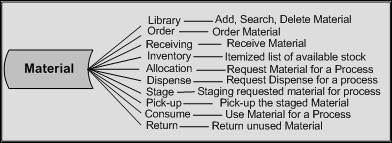
Description of the illustration ch1_04.jpg
Equipment Management enables you to add, modify, search, and view equipment. Equipment can have contaminants that can affect the process. It is crucial to track the state of readiness of the equipment by performing various activities on it, such as cleaning, calibrating, and reserving.
For details, see Agile Recipe Management for Pharmaceuticals Material Management Guide.
Process Management aims at creating a Work Request, using information derived from a Recipe. A Work Request ultimately results in the material being made and placed into Inventory.
A Process can have several components - Product, Project, Campaign, Process Steps, Control Recipe and Work Request. Within a Project, you can have several Campaigns which are small projects to track and make materials. For each campaign, you can create Process Steps, which can result in several lots being made. Each lot is planned using a Control Recipe and eventually completed with a Work Request. For details, see Agile Recipe Management for Pharmaceuticals Material Management Guide.
Standards enable you to create and maintain a library of predefined statements relating to safety, caution, and other general instructions for a manufacturing process. They can be embedded as variables in the text or instructions within a Recipe Action. You can also resolve these variables to a specific standard based on project or campaign requirement. It also comprises of acronyms and abbreviations used in the industry or organization.
Environmental conditions enable you to track compliance against mandated environmental requirements of regulatory agencies.
You can use environment conditions to ensure that the container of material is stored as per recommendation. For example, ensuring that material sample is stored in a specified environment to prevent its deterioration.
Organization Management enables you to identify and associate RMW objects to geographical entities. An Organization houses multiple Companies, a Company is made of one or more Sites, and one or more Locations make a Site.
Throughout the system, the Site entity is used for receiving materials (into a location), for planning the details within a control recipe (identifying the location from which you can consume material), and completing the work request (identifying the location where you can place the newly made material).