Clinical Protocol Management with Deviation Summarization
Overview
As clinical trials become more complex, managing protocol deviations efficiently is key to ensuring data integrity and compliance. When deviation records in Siebel Clinical Trial Management Systems (CTMS) are entered in free-text fields, they often become verbose and difficult to manage. To address this, a Generative AI-based solution is proposed that parses and summarizes protocol deviation records. This system presents the summarized information to the user for review and editing, ensuring accuracy and clarity. Once finalized, the summary is automatically applied to the parent protocol record, reducing manual input and improving overall protocol management efficiency. This approach leverages AI to streamline the process and ensure precise documentation.
Introduction
In Clinical Trial Management Systems (CTMS), managing protocol deviations can be a challenging and labor-intensive process. Detailed deviation descriptions and multiple records often result in verbose entries that are difficult to interpret and summarize. Introducing a Generative AI-based solution for protocol deviation summarization significantly streamlines this process, enhancing clarity and efficiency.
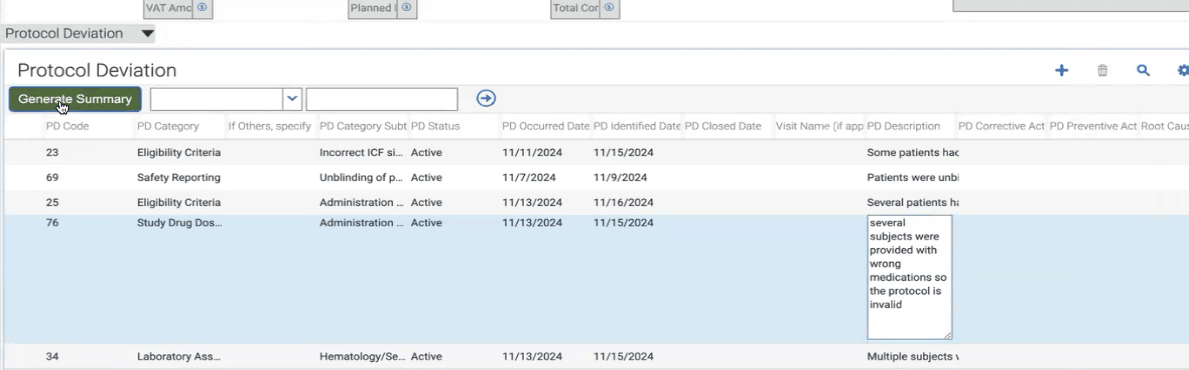
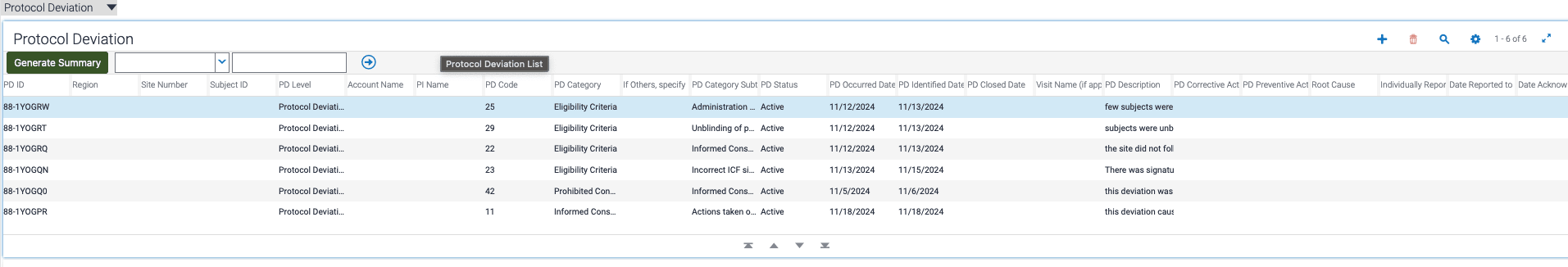
For example, when protocol deviation records are entered into the CTMS, the Clinical Research Associate (CRA) may need to go through the entire older deviation records for the same Protocol record which is very time consuming. But using this system the CRA can click on "Generate Summary" button in the protocol deviation records list applet, Gen AI model parses through the detailed descriptions of each Protocol Deviation, identifies key points, and generates a concise summary. This summary is then presented to the user for review and editing, allowing for interactive refinement. Once approved, the final summary is stamped onto the parent protocol record, ensuring seamless integration with minimal manual intervention.
Challenge: Streamlining Protocol Management with Effective Deviation Summarization
Managing protocol deviations in clinical trials is a complex and critical task. The lack of a streamlined summarization process creates inefficiencies that can compromise data integrity, increase compliance risks, and slow down trial timelines.
Key Issues:
- Managing protocol deviations in clinical trials is inherently complex due to the volume and variability of data involved. Without a streamlined summarization process, teams often face inefficiencies that lead to compromised data integrity, increased compliance risks, and significant delays in trial timelines.
- Deviation descriptions are typically written in free-text formats by different personnel, resulting in verbose, inconsistent, and unstructured entries. This lack of standardization makes it difficult for teams to quickly interpret the critical details needed for timely decision-making and reporting.
- Over the course of a clinical trial, multiple deviation records can accumulate for a single protocol, each capturing separate events or issues. Without a centralized and automated way to summarize and track these records, it becomes increasingly challenging to monitor trends, assess protocol impact, and maintain audit readiness.
Solution:
Key Benefits:
- A generative AI-based summarization system can intelligently process lengthy and complex deviation records, identify key information such as deviation type, affected processes, and corrective actions, and extract essential insights automatically—eliminating the need for manual review and interpretation.
- Automated summarization significantly reduces the risk of human error by consistently applying structured logic to deviation data, ensuring that summaries are accurate, up-to-date, and compliant with regulatory standards at all times.
- By eliminating repetitive manual tasks involved in summarizing deviations, the system frees up valuable time for clinical teams, allowing them to concentrate on higher-value activities such as root cause analysis, trial optimization, and strategic decision-making.
Workflow Overview
- CRA selects a Protocol record with several Protocol deviation records.
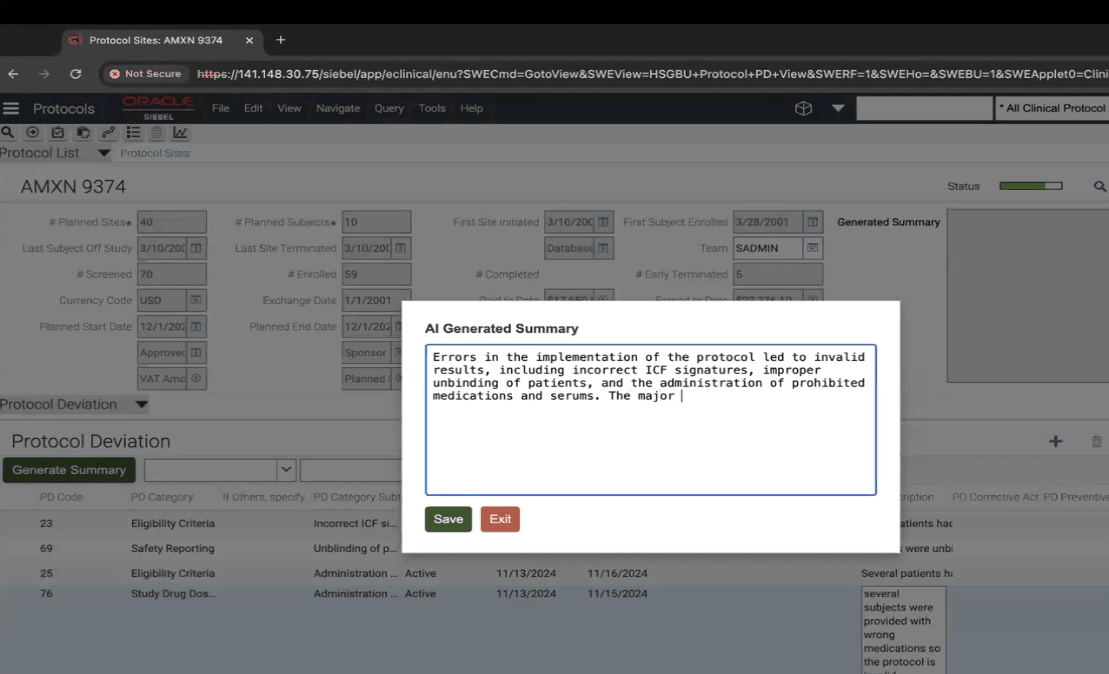
- CRA clicks on Generate Summary Button and gets a Popup of AI Generated Summary of all the Protocol deviations with option to Make required changes.
- CRA saves the Summary for that particular Protocol deviation record.
Key Technologies Involved
- Siebel AI Framework: Siebel AI framework is enabled in the Siebel CTMS Env which provides Gen AI REST Endpoint.
- CTMS UI Customization: SIebel Open UI PR file for UI Customizations, Protocol Deviation records collection and Business Service invocation.
Implementation Strategy
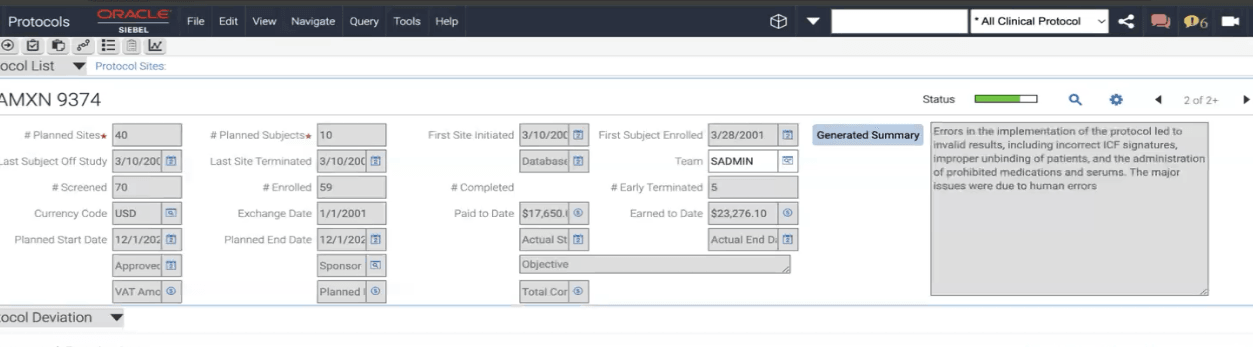
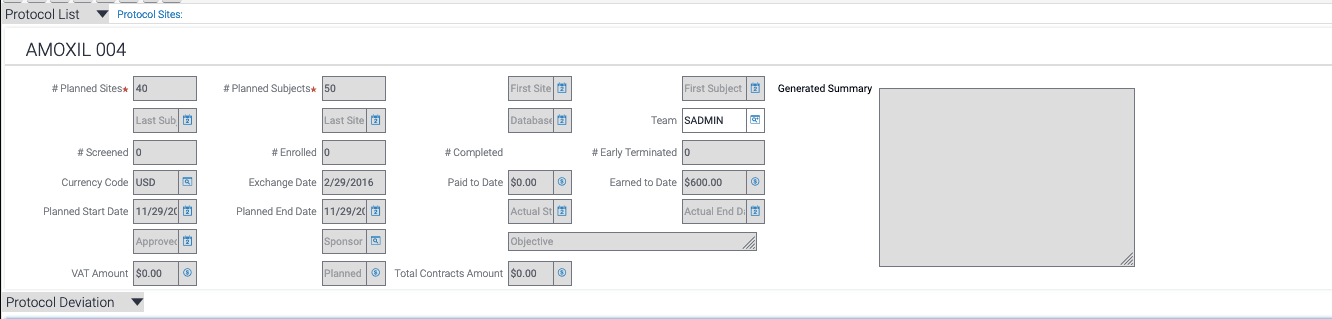
- Adding a new Text Field Area in Clinical Protocol Business Object - "Generated Summary"
- PR File for collecting protocol deviation, invoking Siebel AI Framework REST Endpoint and UI Customization
Architecture Diagram
This functionality can be implemented in 2 ways:
- Leveraging Siebel OCI AI Framework. (Recommended)
- Creating a wrapper Node JS Service that calls the OCI APIs
Leveraging Siebel OCI AI Framework. (Recommended)
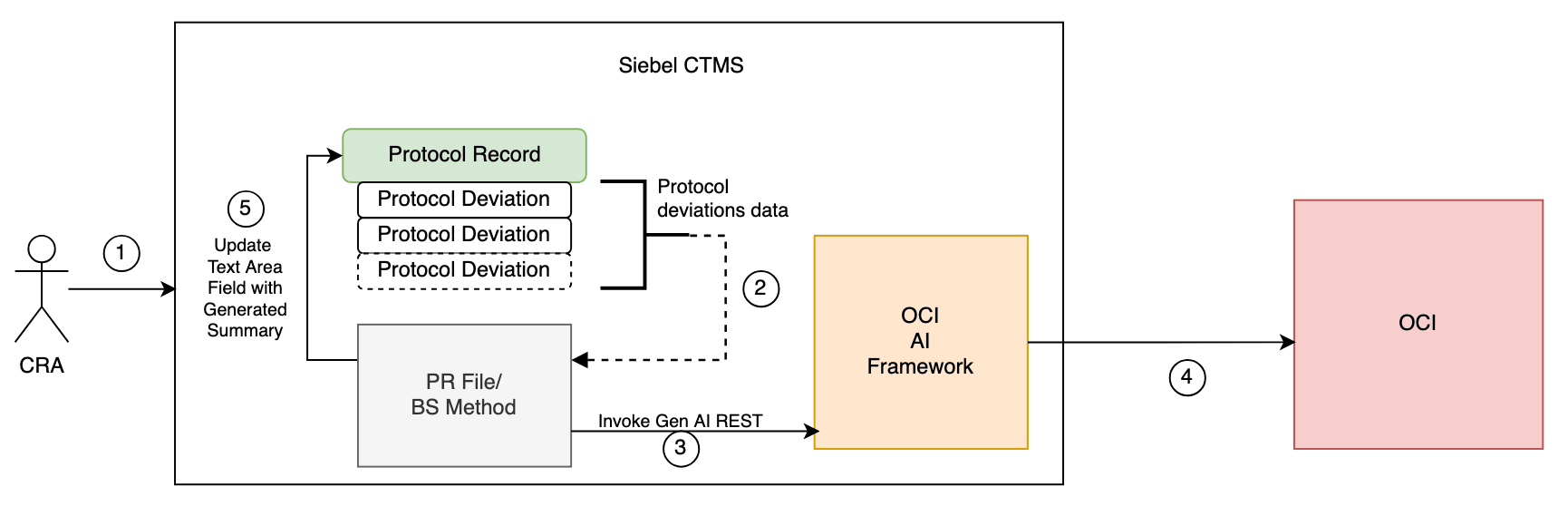
- CRA Clicks on Generate Summary Button on the Protocol deviations list page Applet.
- PR File or BS Scripts collects all the Protocol Deviation records description data.
- OCI AI Framework - Gen AI Endpoint is invoked and the Collective Protocol deviation records data is passed.
- OCI AI Framwork calls the OCI Gen AI and gets the summary.
- PR File/ BS Scripts update the Protocol Records Text Area Field with generated Summary.
Leveraging OCI Gen AI Service via External Node JS Service
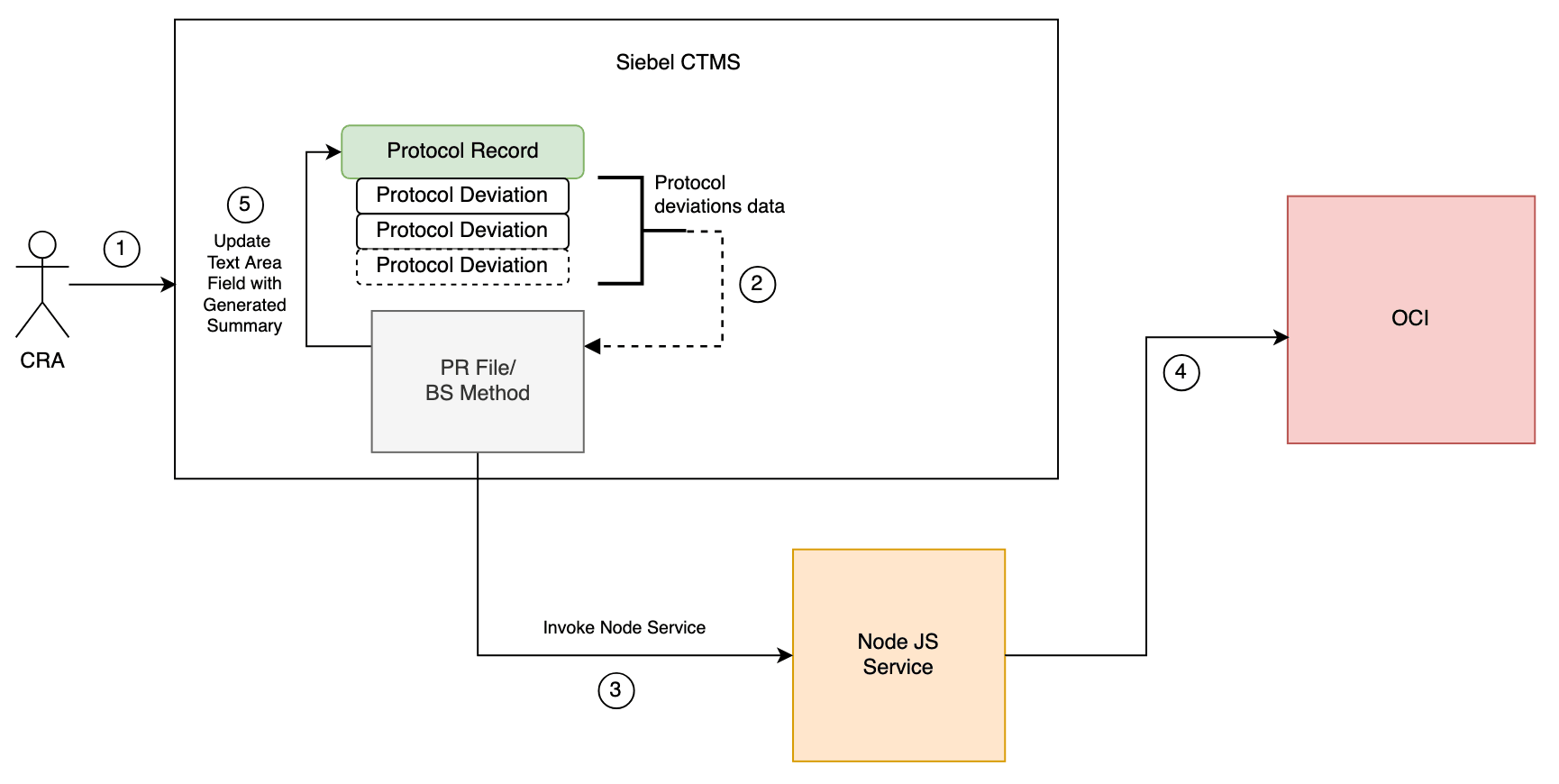
- CRA clicks on the "Generate Summary" button on the Protocol Deviations list page applet.
- PR File or Business Service (BS) scripts collect all protocol deviation record descriptions.
- The external Node.js service is invoked, and the collected protocol deviation data is passed to it.
- The Node.js service calls the OCI Gen AI endpoint and retrieves the generated summary.
- PR File or BS scripts update the Protocol Records text area field with the generated summary.
Siebel Customisations
- Add a button to Applet "HSGBU Site Subject PD List Applet".
- Add a Text Area Field for Clinical Protocol BC and in Applet "Clinical Protocol Short Form
Applet".
- In order to handle Generate Summary action we can take 2 approaches.
- Writing Business Service Method that gets invoked on clicking "Generate Summary" Button. (Recommended)
- PR File registers event listener to handle Button click and execute the Summary generation
and UI Changes.
PR File: Event Listener code
Functionality
The system enables CRAs to generate concise summaries of multiple protocol deviation records with a single click. When the user triggers the summarization, the application collects all deviation descriptions, invokes an external Generative AI service, and automatically updates the protocol management system with the generated summary. This streamlines documentation and ensures consistency across records.
Demo Steps
- CRA clicks on "Generate Summary Button".
![Activity Created]()
- CRA gets a popup window with an option to edit and make changes to generated Summary.
![Activity Created]()
- CRA Saves the Summary after making necessary changes and it gets saved in the Text Area
field.
![Activity Created]()
Benefits
- Streamlined Protocol Management: By automating the summarization of verbose protocol deviation records, the system reduces manual effort, allowing clinical teams to save time and focus on critical trial activities.
- Enhanced Data Accuracy: The AI-powered summarization ensures that only accurate and relevant details are captured, minimizing errors and inconsistencies across protocol documentation.
- Improved User Efficiency: With an intuitive review and editing interface, users can quickly refine and approve summaries, ensuring a seamless workflow and reducing cognitive load.
Future Possibilities and Innovations
- Predict Total % Severity as a BO field in cumulative Protocol deviations.
Notes
- A limitation of the AI-driven summarization feature is that the generated summary may miss crucial deviations.
Conclusion
By leveraging Generative AI for protocol deviation summarization, CRA can significantly enhance the efficiency and accuracy of clinical trial management. The seamless integration of AI-driven summarization reduces manual effort, ensures consistency in documentation, and improves compliance. This innovative approach streamlines protocol deviation handling, enabling Clinical Research Associates to focus on critical decision-making while maintaining data integrity. Automating summary generation and integration into protocol records not only saves time and costs but also enhances the overall efficiency of clinical trial workflows, ensuring more effective and transparent protocol management.
Get Your Copy of the Whitepaper Here!
Excited to explore this innovation? Get all the details in this recipe!
For more insights or design partnerships, reach out to us at siebel_coe_grp@oracle.com