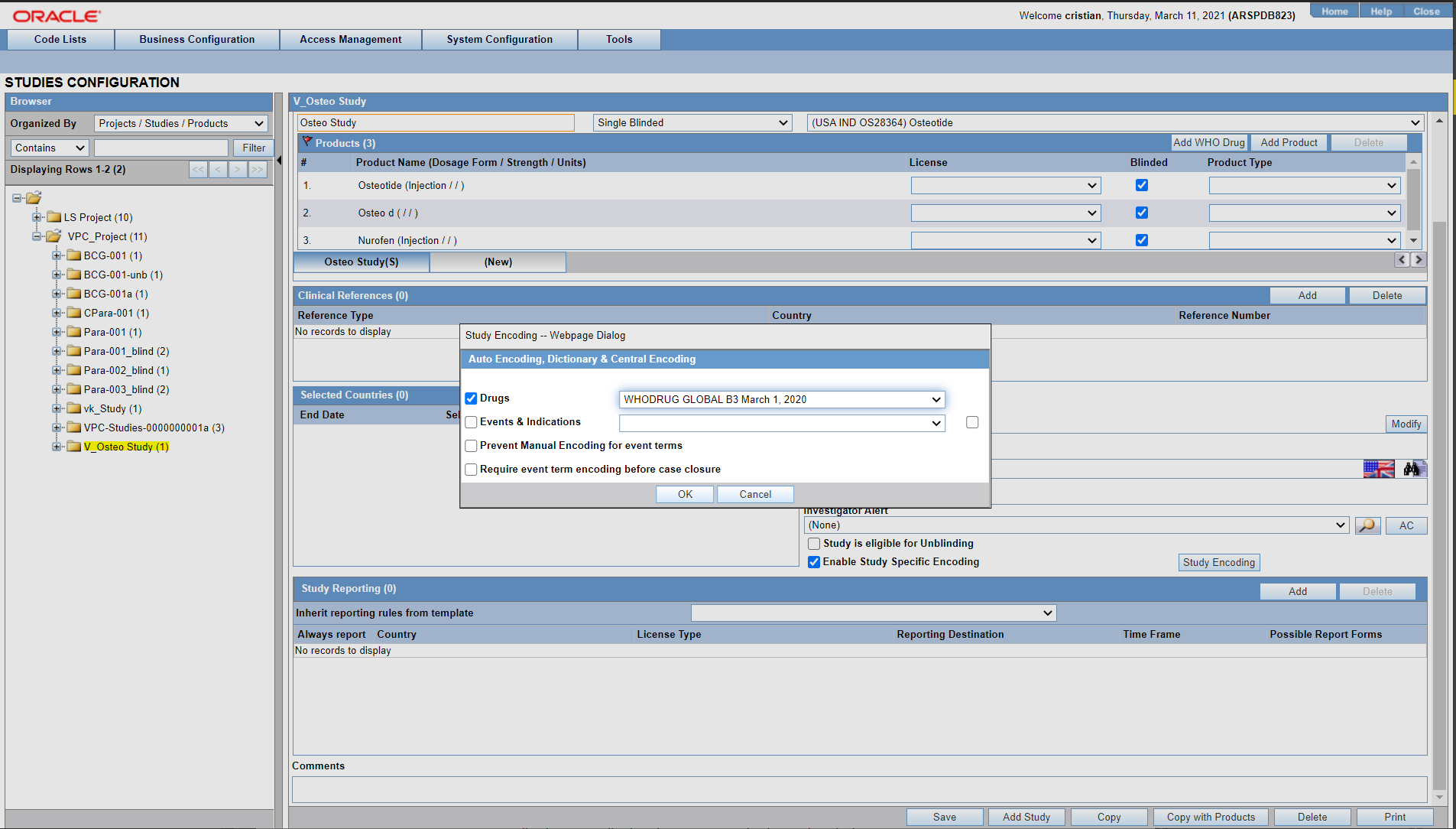
Configuring Auto Encoding
The Auto Encoding features helps you to configure your own dictionary of encoded data. Using this enables you to:
- Configure studies to use dictionaries different from the dictionaries configured using the Case Form configuration.
- Retrieve coded Events, Drugs and Events & Indications and codes from the lists associated with this section.
- Ensure that the expedited reports display the correct verbatim and coded terms.
Use the following procedure to configure Auto Encoding.
Parent topic: Configuring Clinical Studies