Configuring Clinical Studies
It is important to configure clinical studies in the Argus Console because it helps the system categorize the source of information for the cases that have been registered. This screen helps in capturing Study information (study specifics, products involved in the study licensed countries associated with the study and the clinical references used in the expedited reports.
Configuration of the product is done using the Business Configuration > Studies section.
When configuring clinical studies, be aware of the following:
- The IND Reference Number drop down displays only those reference numbers (license
numbers) associated with a product with a License Type of Investigational.
- Console > Business Configuration > Studies > Clinical Reference section (in middle of screen).
- The Reference Number drop down field should be limited to IND (Investigational) US Licenses Number only.
- The Study Name on the Study Configuration can be a maximum 70 Characters (same as the Product name)
When configuring clinical studies, be aware of the following:
- The Study screen has a J Data Entry button that is available only to J users.
- When you click the J Data Entry button, the system presents
a message telling you to enter data in the required fields (Study ID, Project
ID, and at least one (1) Product) before opening the Japanese translation
window.
The Study Type value displays in Japanese and can be one of the following:
- Single Blinded
- Double Blinded
- Not Blinded
-
The License Type value displays in Japanese and can be one of the following:
- Investigational Drug
- Investigational Device
- Investigational Vaccine
- Marketd Drug
- Marketed Device
- Marketed Vaccine
- When you click Add J Drug, the system opens the J WHO Drug Lookup Window to enable the user to add a J Drug. The system adds the drug to the product section of the pop-up.
- The WHO Encoded column has an Encode button. Click this button to open the WHO Drug browser. This enables you to associated the corresponding WHO drug with the J Drug if it is available. The associated WHO drug appears in the product section of the English screen.
- Before closing the J pop-up, the system reminds the user to associate corresponding WHO drugs with the J drugs.
- All error messages are in English.
- After saving the data in the pop-up window, the system makes the same changes on the English screen.
- In browser view, the system displays the Englich product name by default. If the English product name is not availabe, the system displays the Japanese product name.
- Clicking Save closes the window and saves the data, if all J drugs are WHO encoded. If they are not WHO encoded, the system presents the following message: Some J drugs are missing WHO drug association. Do you still want to close the J Data Entry dialog?
- Spell check is notavailable in the Study description dialog.
- When you click Auto Encoding on the J-popup, the system opens
the Auto Encoding dialog.
The following fields appear in the dialog box:
- Drugs
- Events & Indications
- Prevent manual encoding for event
- Require Events & Indications encoding before case closure
- When you click the Centers - Modify button, the system opens
the Modify Study Center dialog box.
This dialog box contains the following fields:/controls
- Center
- Selected Center
- Add
- Add All
- Delete All
- Delete
- OK
- Cancel
- When you copy data, the system does not copy the equivalent Japanese data.
- When you copy data, the application puts Copy of at the beginning of the data in the Name field.
- You can save a duplicate last name if the English ID is unique. If the ID is not unique, the system presents the following message: A duplicate Study ID already exists!
- If the Japanese name is not unique, the system presents the following message: A duplicate ID already exists!
- When navigating from the English screen to the J pop-up for an existing study:
- When you click the J Data Entry button, the system prompts you to save the data on the English screen before opening the J screen: Do you want to save the changes before opening the Japanese screen?
- If you choose to save the data, the system saves the data and the changes appear on the J screen.
- If you choose not to save the data, then changes made on the English screen will not appear on the J screen.
- When entering a new study:
- When you click the J Data Entry button, the system asks you to save the data on the English screen before opening the J screen. The message is: Data must be saved before opening the Japanese screen. Do you want to save?
- If you choose to save the data, the system saves the data and the equivalent data appears on the Japanese screen.
- If you choose not to save the data, the system will not open the J screen.
The following illustration shows the fields associated with this section.
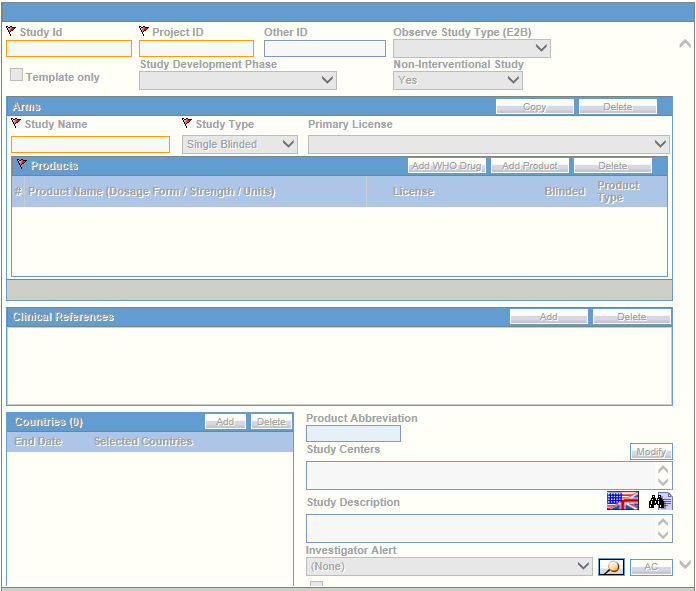
For more information, see: