Complex Examples
The following examples show how multiple rules interact:
- Defining Multiple Paths Through a Trial
- Enabling DCIs in a Conditional Interval
- Defining Multiple Conditional Pretreatment Intervals and DCIs
Parent topic: Flexible Study Design Examples
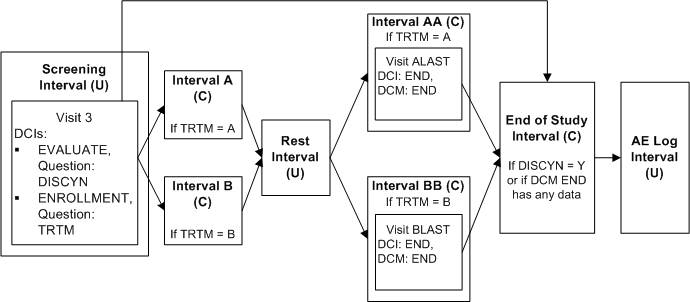
Defining Multiple Paths Through a Trial
This trial includes two treatment paths:
-
Screening Interval, Interval A, Rest Interval, Interval AA, End of Study Interval, AE Log Interval
-
Screening Interval, Interval B, Rest Interval, Interval BB, End of Study Interval, AE Log Interval
Both paths share the Screening, Rest, End of Study Intervals, and AE Log. In addition, if certain criteria are not met the path goes directly from the Screening Interval to the End of Study Interval.
To implement this design in Oracle Clinical:
-
Define Intervals Screening, A, B, Rest, AA, BB, End of Study and AE Log in this order in the study schedule.
-
Define DCI EVALUATE with Question DISCYN with a DVG with values Yes and No, and assign it to Visit 3 in Screening Interval.
-
Define DCI ENROLLMENT with Question TRTM with a DVG with values A and B and assign it also to Visit 3 in Screening Interval.
-
Define DCI END with DCM END and assign it to Visit ALAST in Interval AA and Visit BLAST in Interval BB.
-
Define Interval rule 1 with action "Enable" as follows:
-
Trigger: DCI ENROLLMENT, Question TRTM, value A, CPE Visit 3
-
Target: Interval A, Interval AA
-
-
Define Interval rule 2 with action "Enable" as follows:
-
Trigger: DCI ENROLLMENT, Question TRTM, value B, CPE Visit 3
-
Target: Interval B, Interval BB
-
-
Define Interval rule 3 with action "Enable" as follows:
-
Trigger: DCI END, DCM END [Any Data], (Question null, value null), CPE ALAST, BLAST
-
Target: Interval End of Study
-
RDC Onsite displays the Screening, Rest, and AE Log Intervals and all their unconditional DCIs and the CPEs that contain them, for each patient assigned to the DCI Book, before any data is entered. If you prefer not to display the Rest and AE Log Intervals until they are the next expected Interval, you can define a rule with a trigger in the preceding Interval and a target of [Next Interval].
If the response to DISCYN is N and the response to TRTM is A, all Intervals in path A become expected for the patient and RDC Onsite displays all the unconditional DCIs in Intervals A and AA and the Visit(s) that contain them. If the response DISCYN is N and the response to TRTM is B, the system does the same for path B. In both cases, the End of Study Interval becomes expected for the patient when DCM END has any saved data.
However, if the response to DISCYN is Y, the patient proceeds immediately to the End of Study Interval even a value of A or B is entered for TRTM. None of the other Intervals is either expected or displayed in RDC Onsite.
Note:
You can define an Enable Interval rule triggered in Interval AA and BB even though a Bypass rule exists that bypasses Intervals AA and BB when triggered.
The Rest and AE Log Intervals are automatically expected for patients on both paths because they are defined in that order in the study schedule and are not the target of any rule.
Parent topic: Complex Examples
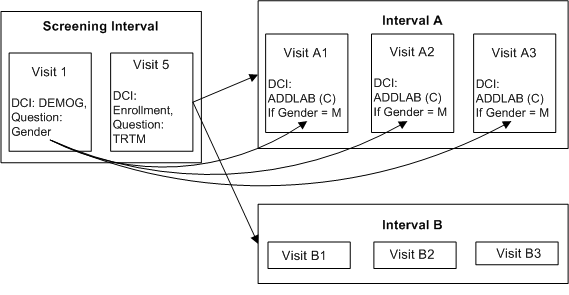
Enabling DCIs in a Conditional Interval
This trial has two treatment paths. In path A only, male patients are required to have an extra blood draw. Both male and female patients are assigned to both treatment paths.
Figure A-9 Conditional DCIs in a Conditional Interval
Description of "Figure A-9 Conditional DCIs in a Conditional Interval"
To implement this design in Oracle Clinical:
-
Define Intervals Screening, A and B.
-
Define DCI DEMOG with Question GENDER with a DVG with values M and F, and assign it to Visit 1 in the Screening Interval.
-
Define DCI ENROLLMENT with Question TRTM with a DVG with values A and B, and assign it to Visit 5 of the Screening Interval.
-
Define DCI ADDLAB and assign it to Visits A1, A2, and A3 in Interval A.
-
Define Interval Rule 1 with action "Enable" as follows:
-
Trigger: DCI ENROLLMENT, Question TRTM, value A, CPE Visit 5
-
Target: Interval A
-
-
Define Interval Rule 2 with action "Enable" as follows:
-
Trigger: DCI ENROLLMENT, Question TRTM, value B, CPE Visit 5
-
Target: Interval B
-
-
Define DCI Rule 3 of type "Enable Across CPEs" as follows:
-
Trigger: DCI DEMOG, Question Gender, value M, CPE Visit 1
-
Target: DCI ADDLAB
-
At Visit 1, the response to the trigger Question GENDER is entered. However, no DCIs become expected for the patient even if the trigger response (M) is entered because the patient is not yet assigned to either Interval A or B. After the response to Question TRTM is entered for Visit 5 either Interval A or B becomes expected for the patient. If TRTM's value is A and GENDER's value is M, then DCI ADDLAB becomes expected in Visits A1, A2, and A3 in Interval A. Only then does RDC Onsite display both Interval A with all its unconditional DCIs and the CPEs that contain them, and DCI ADDLAB for male patients.
Note:
Target DCIs can be contained in multiple CPEs in an Interval that is made expected by an Interval rule triggered by a DCI in a CPE later than the CPE that contains the DCI rule trigger.
Parent topic: Complex Examples
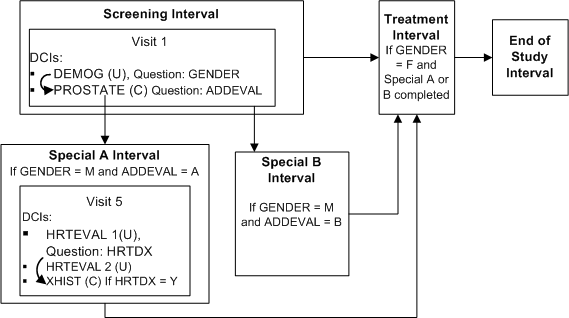
Defining Multiple Conditional Pretreatment Intervals and DCIs
This trial has a single treatment path that includes a Screening, Treatment, and End of Study Interval. In addition, all male patients must complete a PROSTATE DCI during the Screening Interval and one of two additional Intervals (SPECIAL A or SPECIAL B) based on a response in the PROSTATE DCI. Patients in SPECIAL A must also complete a XHIST DCI if they have a history of heart disease.
Figure A-10 Multiple Conditional Pretreatment Intervals and DCIs
Description of "Figure A-10 Multiple Conditional Pretreatment Intervals and DCIs"
To implement this design in Oracle Clinical:
-
Define Intervals Screening, Special A, Special B, Treatment, and End of Study in that order in the study schedule
-
Define DCI DEMOG DCI with Question GENDER with a DVG with values M and F, and assign it to Visit 1 in the Screening Interval
-
Define DCI PROSTATE with Question ADDEVAL with a DVG with values A and B, and assign it to Visit 1 in the Screening Interval
-
Define DCI HRTEVAL1 with Question HRTDX with a DVG with values Y and N, and assign it to Visit 5 in Interval Special A
-
Define DCI XHIST and assign it to Visit 5 in Interval Special A
-
Define Interval Rule 1 with action "Enable" as follows:
-
Trigger: DCI DEMOG, Question GENDER, value F, CPE Visit 1
-
Target: Treatment Interval
-
-
Define DCI Rule 2 of type "Enable Within CPE" as follows:
-
Trigger: DCI DEMOG, Question Gender, value M, CPE Visit 1
-
Target: DCI: PROSTATE
-
-
Define DCI Rule 3 of type "Enable Within CPE" as follows:
-
Trigger: DCI HRTEVAL 1, Question HRTDX, value Y, CPE Visit 5
-
Target: DCI: XHIST
-
When a value of M is entered for Question Gender at Visit 1, the PROSTATE DCI becomes expected for the patient and must be collected at the same visit. If the value of Question ADDEVAL in the PROSTATE DCI is A, Interval Special A becomes expected for the patient. If the value of ADDEVAL is B, Interval Special B becomes expected. Patients in Interval Special A must complete the HRTEVAL DCI, and if their response to Question HRTDX is Y, they must also complete DCI XHIST.
Male patients proceed to the Treatment Interval after completing either Interval Special A or Interval Special B because it is defined next in the study schedule. Female patients proceed to the Treatment Interval immediately after completing the Screening Interval. The Screening and End of Study Intervals are expected and displayed in RDC Onsite for all patients from the beginning of the trial.
RDC Onsite displays Intervals Special A and Special B and the conditional DCIs within them only when they become expected for each patient.
Parent topic: Complex Examples