Basic Examples
Following are examples of how to set up a flexible study in Oracle Clinical by defining DCI Book rules. In the diagrams:
-
Only the setup directly relevant to the example is described. In a real trial, all Intervals would have at least one CPE and all CPEs would have at least one DCI, for example. In addition, each DCI must contain at least one DCM, which must contain at least one Question Group, which in turn contains at least one Question.
-
(U) means unconditional and (C) means conditional. By definition, a conditional DCI or Interval is the target of a rule, and an unconditional DCI or Interval is not.
For more information, see:
- Enabling a DCI within a Clinical Planned Event
- Enabling DCIs Across Clinical Planned Events
- Enabling Intervals Based on the Collection of Any Data
- Enabling Intervals Based on a Data Value
- Skipping to an Interval
- Enabling the Next Interval
Parent topic: Flexible Study Design Examples
Enabling a DCI within a Clinical Planned Event
During the treatment phase of a trial, a physical exam is required at each Clinical Planned Event if and only if the patient's vitals have changed since the last Clinical Planned Event.
Figure A-1 Conditional DCI within a Clinical Planned Event
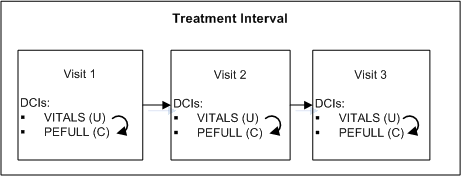
Description of "Figure A-1 Conditional DCI within a Clinical Planned Event"
To implement this design in Oracle Clinical:
-
Define two DCIs: VITALS and PEFULL and assign them to every CPE in the Treatment Interval.
-
At the end of the VITALS DCI, define Question PE_CHANGE asking "Have there been any changes to vitals since the last visit?" with a Yes/No Discrete Value Group.
-
Define a DCI rule of type "Enable DCI Within CPE" with PE_CHANGE as the trigger Question, Yes as the trigger value, and PEFULL as the target DCI.
At each Clinical Planned Event, the response to the PE_CHANGE Question will determine whether the PEFULL DCI is required for the patient; if Yes, the exam is expected and RDC Onsite displays the corresponding CRF for the patient. If No is entered, it is not expected.
Note:
One rule covers every case where the VITALS and PEFULL DCIs occur in the same CPE.
Parent topic: Basic Examples
Enabling DCIs Across Clinical Planned Events
A trial has a substudy that requires an extra blood draw at Visits 1, 2, and 3 of the Treatment phase. Not all patients should participate in the substudy.
Figure A-2 Conditional DCI Across Clinical Planned Events
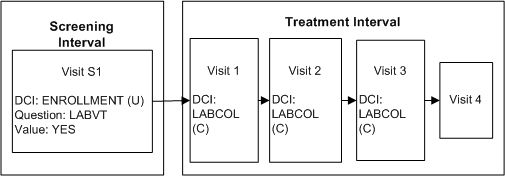
Description of "Figure A-2 Conditional DCI Across Clinical Planned Events"
To implement this design in Oracle Clinical:
-
Define DCI ENROLLMENT and assign it to Visit S1 in the Screening Interval.
-
Define Question LABVT in DCI ENROLLMENT with a Yes/No Discrete Value Group.
-
Define DCI LABCOL to request the blood draw and record the lab results, and assign it to Visit T1 in the Treatment Interval.
-
Define a DCI rule of type "Enable DCI Across CPEs" with LABVT as the trigger Question, Yes as the trigger value, and LABCOLL as the target DCI.
If a value of Yes is entered for Question LABVT, DCI LABCOLL becomes expected for the patient at every CPE to which it is assigned. RDC Onsite displays the corresponding CRF in visits that are currently expected. If a value of No is entered, DCI LABCOLL is never expected for the patient.
Note:
One rule enables DCI LABCOLL in every CPE to which it is assigned. If you want the same blood test to be performed but without being conditional on the response to Question LABVT, copy the DCI, rename it, and assign the renamed DCI to the appropriate CPEs. If you want it to be conditional upon a different Question response, define another rule. If you want it to be performed on all patients unconditionally, do not define any rule with it as target.
Parent topic: Basic Examples
Enabling Intervals Based on the Collection of Any Data
All patients in a trial have the same visit schedule expected, going from the screening Interval to the treatment Interval, to the end-of-study Interval.
Figure A-3 Enable Intervals Based on Any Data
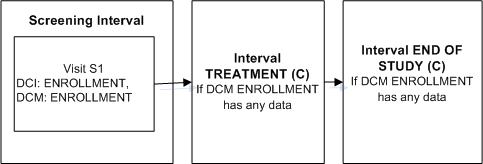
Description of "Figure A-3 Enable Intervals Based on Any Data"
To implement this design in Oracle Clinical:
-
Define DCI ENROLLMENT that includes DCM ENROLLMENT and assign it to Visit S1 in the Screening Interval.
-
Define Intervals TREATMENT and an END OF STUDY.
-
Define an Interval rule with action "Enable" with DCM ENROLLMENT as the trigger and [Any Data] as the trigger value, and Intervals TREATMENT and END OF STUDY as the target.
As soon as an operator enters any data for a Question in DCM ENROLLMENT and saves, Intervals TREATMENT and END OF STUDY and all their CPEs and unconditional DCIs become expected for the patient. RDC Onsite displays all corresponding expected CRFs and all visits that contain expected CRFs.
Note:
An Interval rule with action "Enable" can make one or more Intervals expected for a patient.
Parent topic: Basic Examples
Enabling Intervals Based on a Data Value
Patients in a trial must be assigned to one of two treatment arms depending on their response to a Question collected in the Enrollment DCI at the first visit.
Figure A-4 Enable Intervals Based on a Data Value
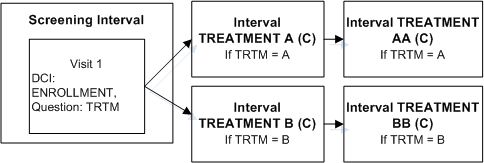
Description of "Figure A-4 Enable Intervals Based on a Data Value"
To implement this design in Oracle Clinical:
-
Define DCI ENROLLMENT and assign it to Visit S1 in the Screening Interval.
-
Define Question TRTM in DCI ENROLLMENT with a Discrete Value Group that includes values A and B.
-
Define two Intervals, TREATMENT A and TREATMENT B.
-
Define an Interval rule with action "Enable" with TRTM as the trigger Question, A as the trigger value, and Intervals TREATMENT A and TREATMENT AA as the target.
-
Define an Interval rule with action "Enable" with TRTM as the trigger Question, B as the trigger value, and Intervals TREATMENT B TREATMENT BB as the target.
If a value of A is entered for Question TRTM, Interval TREATMENT A and all its CPEs and unconditional DCIs become expected for the patient. If a value of B is entered for Question TRTM, Interval TREATMENT B and all its CPEs and unconditional DCIs become expected for the patient. RDC Onsite displays all corresponding expected CRFs and all visits that contain expected CRFs.
Note:
An Interval rule with action "Enable" can make one or more Intervals expected for a patient.
Parent topic: Basic Examples
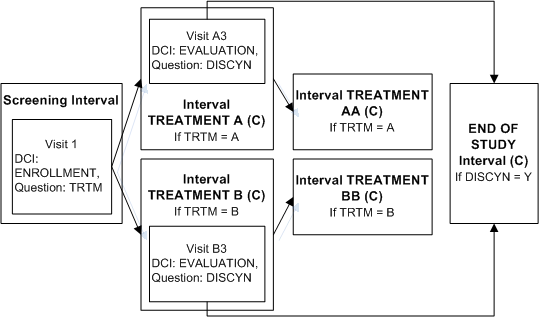
Skipping to an Interval
In the same trial, patients in both treatment arms may need to exit the study after the first treatment Interval. An evaluation DCI must be collected ending with a summary Question to collect the decision to discontinue the patient from the study or not.
To implement this design in Oracle Clinical:
-
Define the Screening Interval and Intervals TREATMENT A, TREATMENT AA, TREATMENT B, TREATMENT BB as in the example Enabling Intervals Based on a Data Value.
-
Define Interval END OF STUDY.
-
Define DCI EVALUATION and assign to Visit A3 in Interval TREATMENT A and Visit B3 in TREATMENT B.
-
Define Question DISCYN in DCI Evaluation with a Yes/No Discrete Value Group assigned.
-
Define an Interval rule with action "Bypass To" with DISCYN as the trigger Question, Yes as the trigger value, and Interval END OF STUDY as the target.
If a value of Yes is entered for Question DISCNY, Interval TREATMENT AA is no longer expected for a patient in TREATMENT A; or TREATMENT BB is no longer expected a patient in Interval TREATMENT B. In both cases Interval END OF STUDY becomes the next expected Interval. RDC Onsite no longer displays either TREATMENT AA or TREATMENT BB but does display all CRFs corresponding to the unconditional DCIs in Interval END OF STUDY and the Visit(s) that contain them.
If a value of No is entered for Question DISCNY, there is no change. The patient completing TREATMENT A proceeds to TREATMENT AA and the patient completing TREATMENT B proceeds to TREATMENT BB. RDC Onsite continues to display all CRFs corresponding to the unconditional DCIs in those Intervals and the Visit(s) that contain them.
Note:
In this trial, you can use any of several methods to ensure that patients who continue to TREATMENT AA or BB also complete the END OF STUDY Interval. For example, define an "Enable" Interval rule with [Any Data] in the first DCM scheduled for the first visit of TREATMENT AA as the trigger and Interval END OF STUDY as the target. Define a similar Interval rule for TREATMENT BB. The diagram then looks like Figure A-6.
An Interval can be the target of two rules if one rule has the action "Enable" and the other has the action "Bypass To."
Figure A-6 Enabling and Bypassing To the Same Interval
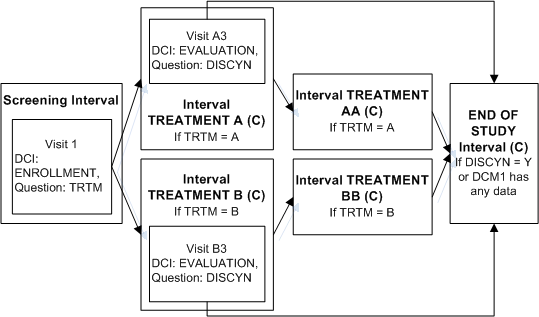
Description of "Figure A-6 Enabling and Bypassing To the Same Interval"
Parent topic: Basic Examples
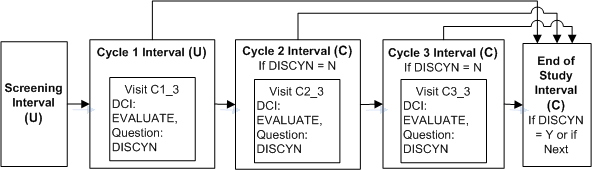
Enabling the Next Interval
A trial requires a screening Interval followed by three cycle Intervals. The cycle Intervals are exactly the same, but each patient will move to the next cycle only if he or she passes an evaluation in the current cycle and the Discontinue? Question has No entered as a response. When the patient completes all three cycle Intervals or fails the evaluation he or she proceeds to the End of Study Interval.
To implement this design in Oracle Clinical:
-
Define the Intervals SCREENING, END OF STUDY, and Interval CYCLE 1.
-
Define DCI EVALUATION and assign to Visit C3 in Interval CYCLE 1.
-
Define Question DISCYN in DCI EVALUATION with a Yes/No Discrete Value Group assigned.
-
Define Intervals CYCLE 1, CYCLE 2, and CYCLE 3. Add their CPEs to the DCI Book, add DCIs to CYCLE 1, then copy those pages to the CPEs in the other cycle Intervals; see Copying Pages for a CPE.
-
Define an Interval rule with action "Enable" with DISCYN as the trigger Question, No as the trigger value, and [Next Interval] as the target.
-
Define an Interval rule with action "Bypass To: with DISCYN as the trigger Question, Yes as the trigger value, and Interval END OF STUDY as the target.
If a value of No is entered for Question DISCNY, the next CYCLEx Interval becomes expected for the patient. If a value of Yes is entered for DISCYN, the END OF STUDY Interval becomes expected for the patient. Until a value is entered for DISCYN, neither the next CYCLE Interval nor the END OF STUDY Interval is displayed in RDC Onsite.
Note:
You must be careful to define more CYCLE Intervals than you will need. If not, every patient in the last defined CYCLE Interval proceeds to END OF STUDY because it is the Next Interval and therefore the target of both the Enable and the Bypass To rules.
If you need to add cycles after entering data, you must define addition Intervals in the study schedule, then set the Book to Provisional and add DCI Book Pages to the Intervals' CPEs, and set the Book's status back to Active.
Parent topic: Basic Examples