Creating Clinical Planned Events
A clinical planned event (CPE) usually corresponds to a visit. You can also define a CPE for the purpose of associating it with DCIs (corresponding to CRFs) that you want to have available in a DCI Book without associating them with a particular visit, such as AEs or ConMeds. If you are defining Processes in Oracle Clinical, you can create a CPE to schedule a Process during an Interval.
If you are working in a flexible study, see Defining Intervals and CPEs for Flexible Studies for more information.
To create a clinical planned event:
- From the Design menu, select Schedule, then select Events. The Study Versions window opens.
- Choose a study version and click Events. The Maintain Clinical Planned Events window opens, displaying all CPEs in the database.
- Insert a row, then enter a title for the event in the Event Name field. The Event Name must be unique within this study version. You may want to take page numbering into consideration when you name your CPEs, especially in a flexible study; see Sample Numbering Schemes.
- Specify the event details:
- Choose a Process Name for the event from the list of
values. A process is a collection of clinical planned procedures
performed at a CPE; a process occurs at each CPE.
The values in this list are populated in the Maintain Processes window.
- Choose an Interval Name from the list of values.
Each CPE occurs during an Interval, and you can choose a phase, period,
sub-period, or the entire study as the Interval for this event.
Note:
When you create a study using Easy Study Design, the system automatically creates a Phase Interval called DEFAULT STUDY PHASE. If you are creating a flexible study you cannot use this default Interval and must explicitly create Intervals.Note:
The system prevents you from changing a CPE's Interval if the study is flexible and has an active DCI Book that contains Interval rules. If the DCI Book is provisional, the system sets its validation status to Pending. When you activate the book or run the validation job explicitly, the job determines if the changes are valid. - Enter a Visit Number. A CPE's visit number must be unique within this study version. The Visit Number for each CPE controls the order in which they occur in the DCI Book and in the study. CPEs with a lower Visit No. cannot occur after a CPE with a higher Visit No., so be sure to give CPEs the right Visit No. relative to one another. You can add a CPE later, but you must renumber all subsequent CPEs to maintain the correct relative order.
- If this event describes the termination visit, choose a Termination Code. Valid codes are "early" or "normal." You can change this field at any time, provided the study has at most one early and one normal termination visit.
- Select the Dispensing Visit? field if you want to indicate that the visit includes the administration of a study drug.
- Populate the two pairs of Minimum/Maximum offset
parameters:
- Offsets from Interval Start mark the event's time frame from the start of the Interval.
- Offsets from Previous Event mark the event's time frame from the last event.
- Enter a Label Code, which is a one-character code to print on the kit label to represent this event.
- (Optional) Enter a Trigger Event, which describes the conditions that must exist before this event can occur.
- Choose a Process Name for the event from the list of
values. A process is a collection of clinical planned procedures
performed at a CPE; a process occurs at each CPE.
- Save.
For more information, see:
Parent topic: Treatments and Schedules
Using CPE Offsets
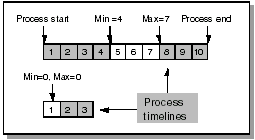
The minimum offset is the minimum number of time units that must elapse before a particular CPE can take place. The maximum offset is the maximum number of time units that can elapse before a particular CPE must take place.
The following diagram shows how to use offsets to specify the time frame within which a planned event should take place:
For a CPE that can occur on day 5, 6, or 7, the minimum offset is 4 and the maximum 7. A CPE on Day 1 has a minimum and maximum offset of zero.
Oracle Clinical reports missing CPEs to facilitate the DCM tracking process. The system automatically calculates the minimum and maximum offset of each CPE from the start of the study.
The system derives the calculation in the following manner:
- The minimum offset from the start of the study is the sum of the minimum duration for the Intervals that occur before the start of the Interval to which the CPE belongs, plus the offset for the CPE within the Interval.
- Similarly, the system calculates the maximum offset based on the maximum duration and offset.
For more information, see:
Parent topic: Creating Clinical Planned Events
Examples of Offsets
Following are examples of calculating when a CPE should occur within a clinical study version.
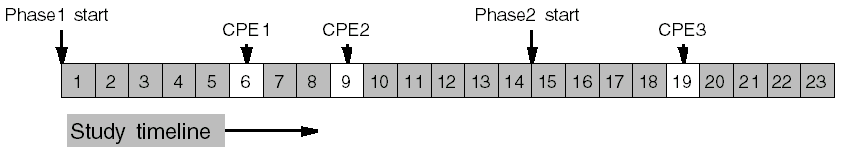
Simple examples:
CPE1 occurs 6 days into the study. CPE2 occurs 9 days into the study. CPE3 occurs 5 days into Phase 2, which comes after the 14 days of Phase 1. So, visit 3 occurs 14 + 5 = 19 days into the study.
Different Minimum and Maximum Example
CPE1 occurs 6 to 8 days into the study. CPE2 occurs 9 to 11 days into the study. CPE3 occurs 5 to 8 days into phase 2, which comes after the 14 days of phase 1. So, visit 3 occurs after a minimum of 14 + 5 = 19 days into the study and by a maximum of 14 + 8 = 22 days into the study.
Offsets from Interval Example
CPE1 occurs 6 days into the study. CPE2 occurs 3 days after visit 1; so visit 2 occurs 6 + 3 = 9 days into the study. CPE3 occurs 5 days into Phase 2, which comes after the 14 days of Phase 1. So, CPE3 occurs 14 + 5 = 19 days into the study.
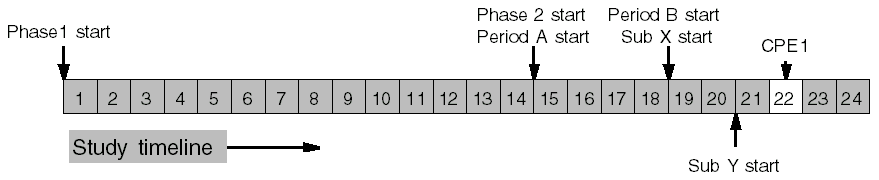
Periods and Sub-periods Example
CPE1 occurs 2 days into Sub-period Y of Period B of Phase 2. Sub-period Y comes after Sub-period X, which is 2 days long. Period B comes after Period A, which is 4 days long. Phase 2 comes after Phase 1, which is 14 days long. So, CPE1 occurs after Phase 1 + Period A + Sub-period X, plus 2 days into Sub-period Y: 14 + 4 + 2 + 2 = 22 days.
It is possible to mix the different cases. For example, minimum and maximum offsets and duration can be combined with periods and sub-periods. Also, an event can always be defined as occurring a minimum and maximum time after the previous event.
Parent topic: Using CPE Offsets