Viewing a Study and Site Summary Report
The one-page Study and Site Summary Report includes up-to-date information on the total number of patients and CRFs in the entire study and at the selected site. The metrics include counts for patients and CRFs at various statuses in the study.
To view and print the Study and Site Summary Report:
- Open the Home, Patient Casebooks, or Reports page.
You can change the study and site before viewing the summary report from the Home or Patient Casebooks page. The Reports page has no option to change the study and site.
- Click the Study and Site Summary link located on the top right of the
page.
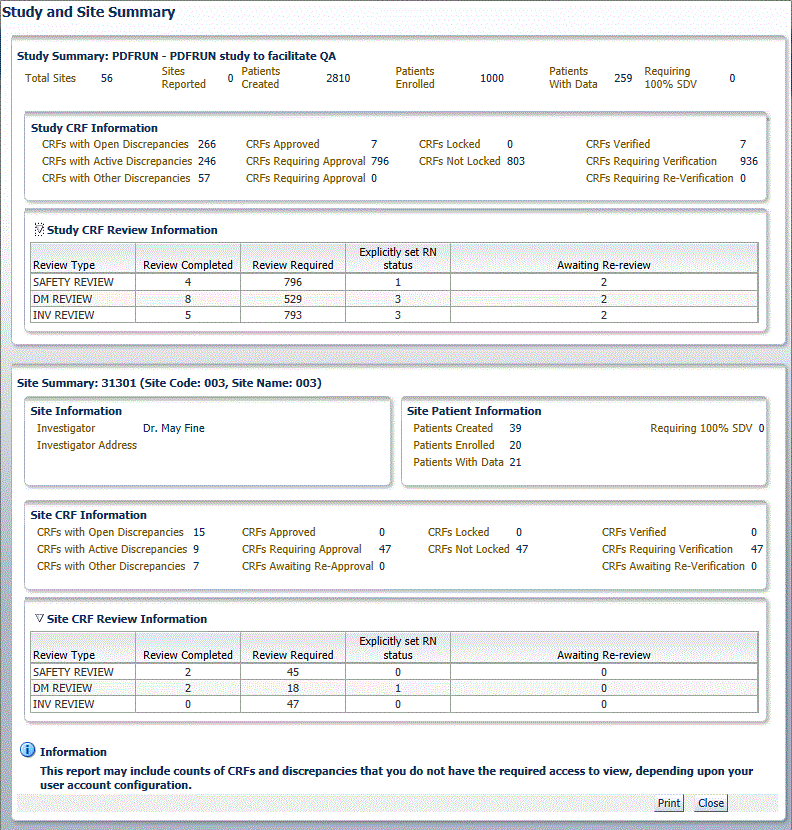
RDC Onsite opens a new window and displays up-to-date metrics for the current study and site. The below image shows a sample Study and Site Summary Report.
- To print the Study and Site Summary Report, click Print. Use a Landscape orientation to format the report properly on the printed page.
- To close the window and return to the previous page, click Close.
For more information, see:
Parent topic: Viewing Summary Reports
About the Study Summary
The Study Summary section lists the overall metrics for the currently selected study. You can view metrics only for the sites to which you have privileges.
- Study Information — Lists the total number of sites in the study, the number of sites in the study for which you have access to view the information, and the number of patients created, patients enrolled, and patients with data entered for the study.
- Study CRF Information — Lists the total number of planned and unplanned CRFs for the study, and the total number of CRFs across the study with various discrepancy, verification, lock, approval statuses, and custom review statuses. Verification and custom review metrics appear only if you have the appropriate privileges.
Parent topic: Viewing a Study and Site Summary Report
About the Site Summary
The Site Summary section lists the overall metrics for the current site.
- Site Information — Lists the address of the site, and the name and address of the site investigator.
- Site Patient Information — Lists the number of patients created, patients enrolled, patients with data entered for the site and patients requiring 100% Source Data Verification (SDV).
- Site CRF Information — Lists the total number of planned and unplanned CRFs for the site, and the sum totals of CRFs with various verification, lock, discrepancy, and approval statuses.
Parent topic: Viewing a Study and Site Summary Report
About the Counts in the Study and Site Summary
- The Study and Site Summary may include counts of CRFs and discrepancies that you do not have the required access to view, depending upon your user account configuration.
- The counts include the CRFs and discrepancies that you do not have the required access to view, if any, depending upon your user account configuration.
- The counts for Planned CRFs include all conditional CRFs that have become expected.
- The CRF counts in the Site Summary section are based on the CRF site assignment. If data entry takes place in two sites for the same patient, RDC Onsite apportions the CRF counts between the two sites.
Parent topic: Viewing a Study and Site Summary Report