Granting Oracle Clinical Users Access to RDC
A user who has access to a study in Oracle Clinical does not automatically have access to that study in RDC unless you use the Study Security form in the RDC Administration application to assign specific privileges to the user.
Alternatively, you can use the DMGR RDC ACCESS short value in the OCL_STATE local reference codelist to automatically grant an Oracle Clinical user access to the studies in RDC.
Users with the Superuser flag selected in the Oracle Accounts form in Oracle Clinical always have access to all studies in both Test and Production modes.
To automatically grant an Oracle Clinical user access to RDC:
- Define the study or studies that the user can access. See the Oracle Clinical Administrator's Guide for more information.
- Open Oracle Clinical.
- Navigate to Admin, Reference Codelists, and then select Local Codelists.
- Query for the OCL_STATE local reference codelist:
- Enter OCL_STATE in the Name field.
- Press F8 to execute the query.
- Scroll to the DMGR RDC ACCESS short value.
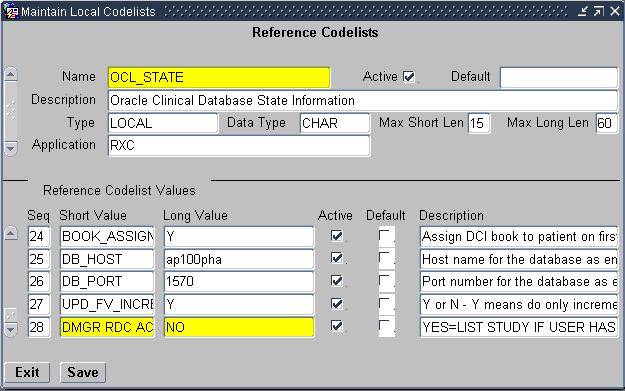
- Set the long value to YES.
- Save your changes.
A user who is granted RDC study access in this manner has all RDC privileges except the APPROVE, BROWSE_VERIFY, and VERIFY privileges. (The UPD_LOCK_OC privilege, which is an Oracle Clinical privilege only, is also excluded). You can restrict such a user's access to RDC by limiting privileges at the study or site level.
When set to YES, a user with no study privileges defined for RDC but with study access defined in Oracle Clinical is automatically given RDC access to the study as well, in both Test and Production modes.
When set to NO, a user granted access to a study in Oracle Clinical does not automatically have access to that study in RDC. You can use the Study Security form in the RDC Administration application to assign specific privileges to the user.
Parent topic: Securing Remote Data Capture