2 Set up clinical data models
A clinical data model is a set of logically related tables. You need one input model for each data source. You create target models to review, analyze, or report data. You use transformations to standardize and merge data from one or more source models into a target model.
Figure 2-1 Example of clinical data model use in a study
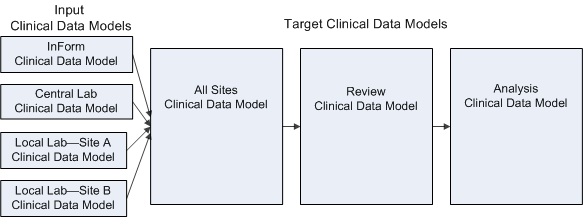
Description of "Figure 2-1 Example of clinical data model use in a study"
The following table lists the tasks you can perform to set up clinical data models.
Table 2-1 High-level steps
| Task | More Information |
|---|---|
|
Create an input clinical data model for every lab. |
|
|
Create an input clinical data model for your clinical data system. |
If you are using InForm, set up a connection to the InForm study development database and load metadata from there to create the clinical data model. See Create a clinical data model for InForm data. If you are using a different clinical data capture system, see Create a file input clinical data model. |
|
Create target clinical data models and tables as needed. |
Create models with tables in the format you need for review and analysis, and any intermediate models required. See Create a target clinical data model for transformed data. You can load metadata from a file using a required syntax, copy a model from another study, create tables manually, or create a study model from a library model, which allows you to update the study model when the library model is updated. To add individual tables, see Add tables. |
|
For each table, define additional attributes and columns. |
Add constraints to tables. A primary key is required. Set up data blinding in tables. Add columns to support filtering. See Use SDTM identifiers to support important functionality. Set up Unit of Work data processing for tables (optional). Configure table display on the Listings page for columns on the Listings pages (optional). Map columns to data to be derived from TMS (in target models, if you are using TMS). |
|
Add a Subject Visit table. |
Add Subject Visit and Subject tables. Each study must have a Subject Visit table with SDTM identifiers in one model. |
|
Install each model. |
|
|
Upgrade each model to Quality Control (optional) and then Production. |
More information on the tasks in this chapter:
- Create a file input clinical data model
- Create a clinical data model for InForm data
- Create a target clinical data model for transformed data
- Add tables
- Add columns to a table manually
- Add constraints to tables
- Set up Unit of Work data processing for tables
- Configure table display on the Listings page
- Set up data blinding in tables
- Add Subject Visit and Subject tables
- Map columns to data to be derived from TMS
- Extract data from a clinical data model
- Modify a clinical data model
- Install a clinical data model
- Unblind and reblind data
- FAQs