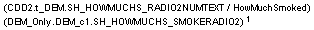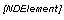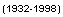Metadata in annotated CRFs
The following table presents how the meta data is displayed in the annotated CRF and in the tables that follow.
Meta Data |
Displayed |
|---|---|
External Column Mappings |
Appear in parenthesis above or beside their corresponding control:
|
External Mapping Companion Table |
Appears labeled as such below the form. A companion table appears for each external mapping table that has form controls mapped to it. For an example, see Data mapping tables. |
Selection/Element Values |
Selection values for check boxes and radio controls in a group control appear in square braces in front of their corresponding controls. If the value is derived from the control definition (for example, the element value of a simple control), the value appears in italic font. Selection value example:
Element values for pulldown controls appear in companion tables below the form. The pulldown control shows the title of the appropriate companion table. Element value example:
|
Date Controls |
Year ranges appear in parenthesis beside their corresponding control:
If a date control component allows an “unknown” value, “Unk” appears in the control:
|
Control/Unit Companion Tables |
Appear below the form. For an example, see Unit lists. |
Design Notes |
|
Item Not Required References |
Denoted by an asterisk in the first column of the form (where the items/itemsets are numbered).
|
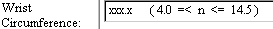
Client-Side Range Checks |
The range check notation is displayed in the control.
|
Electronic Signature Affidavit |
Appears below the form in the Annotated Trial Design view. For an example, Electronic signature affidavits. |
Pulldown List References |
The control contains a label that corresponds to a list in the companion tables following the form. |
Text Box Specifications |
Appear in the control:
|
Cover page |
Appears when you select File, Annotated Trial Design and you have chosen to display it from the Annotated CRF Settings dialog box. |
Sponsor Name |
Part of the cover page displayed when selected on the Annotated CRF Settings dialog box. A suggested use of the Trial Note is to provide a revision number on the cover page. |