Displaying RefName paths for all controls
To define control path mappings by displaying the RefName paths for all controls in the trial:
- Open the Data Mappings window for the Oracle Clinical mapping in which you want to create control path mappings.
- Right-click the Mappings node and select Show All Controls.
The InForm Architect application updates the Data Mappings window with a list of RefName paths for all controls in the trial.
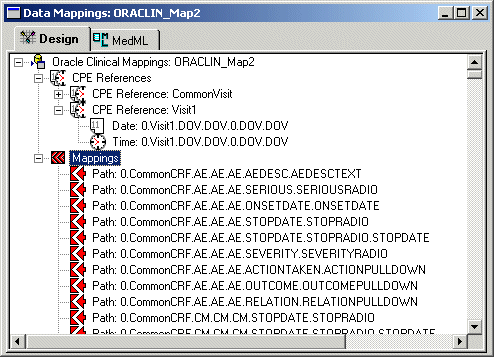
- Select the RefName path of a control, and edit its mapping properties in the Properties window.

The following table describes the properties of a control mapping definition.
Property |
Description |
|---|---|
CPE Reference |
Name of the subevent of the Clinically Planned Event (CPE) with which the data in the control is associated. This name matches the CPE Reference property specified for the CPE Reference mapping definition. REQUIRED.. |
DCI Name |
Data Collection Instrument (DCI) name, a customer-defined grouping of Data Collection Modules. REQUIRED.. |
DCM Name |
Data Collection Module (DCM) name, a customer-defined grouping of data items. REQUIRED.. |
Subset |
Name of a customer-defined grouping by data type. REQUIRED.. |
Question Group |
Name of a customer-defined grouping by question type. REQUIRED.. |
Question |
Customer-defined standard variable name for the question (for example, lab test performed). REQUIRED.. |
Question Value |
Answer to the question, or test result. REQUIRED.. |
Date and Time |
If the field is a datetime field, the pertinent part of the data: Date and Time, Date part only, or Time part only. OPTIONAL |
Output as Uppercase |
True or False, indicating whether the data is translated to uppercase. True is the default. REQUIRED.. |
Repeat Sequence Number |
Number incremented for each result. You can also use a repeat sequence number in conjunction with the Occurrence number used to indicate dependant or related data. A common repeat sequence number indicates a relationship between values. OPTIONAL. |
Occurrence |
Number of test performed; used to associate repeating data, for example repeated labs. A change in occurrence number indicates a retest value (for example, initial value=0, retest value =1). OPTIONAL; default value is zero. |
SubEvent Number |
Number assigned to a particular event (patient, visit and DCM) when the result date or time information does not match the previous date or time for the given event. Use a unique subevent number for each such assignment. OPTIONAL; default value is zero. |
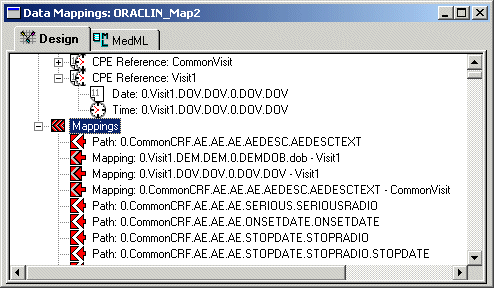
The following figure shows the Data Mappings window after some controls have been mapped to their CPE References. Note that the CPE Reference is appended to the RefName path of mapped controls.