Sample WHODrug Dictionary with Format C or C3
This section provides a structural diagram of the sample WHODrug format C dictionary, and the dictionary level and Level Relations Definitions that are required to create a dictionary with this structure.
For more information, see:
- Sample WHODrug Dictionary with Format C or C3 Structure
- Sample WHODrug Dictionary with Format C or C3 Level Definitions
- Sample WHODrug Dictionary with Format C or C3 Level Relations Definitions
- WHODrug Format C or C3 Data Loaded as TMS Informative Notes
Parent topic: Sample Dictionary Definitions
Sample WHODrug Dictionary with Format C or C3 Structure
The sample WHODrug dictionary structure in Figure A-7 corresponds to the definitions provided in Sample WHODrug Dictionary with Format C or C3 Level Definitions and Sample WHODrug Dictionary with Format C or C3 Level Relations Definitions.
Figure A-7 Figure A-7 Sample WHODrug Dictionary with Format C or C3 Structure
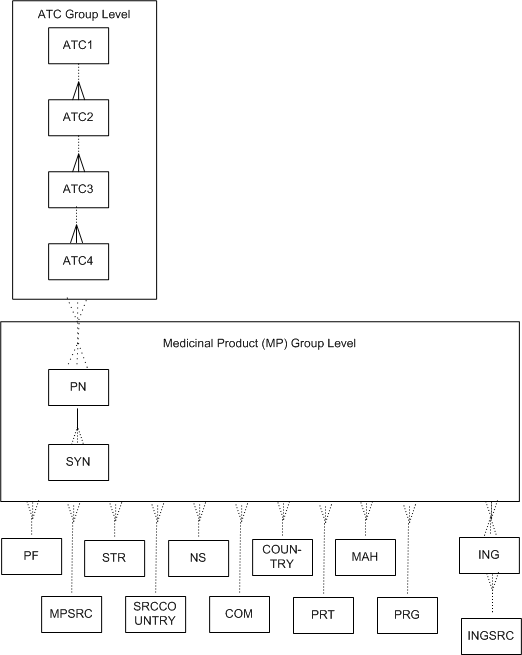
Parent topic: Sample WHODrug Dictionary with Format C or C3
Sample WHODrug Dictionary with Format C or C3 Level Definitions
See Defining the Dictionary Levels for instructions on defining dictionary levels using the Define Dictionaries window in TMS.
Table A-13 Sample WHODrug C or C3 Dictionary Level Definitions
| Short Name | Name | Classifi-cation Level? | Report Value? | Level Order | Level Type | Term Unique? | Group Short Name |
|---|---|---|---|---|---|---|---|
|
ATC |
Anatomical-Therapeutic-Chemical Classification Group |
0 |
Group Level |
None |
|||
|
ATC1 |
ATC 1 |
X |
0 |
Level |
None |
ATC |
|
|
ATC2 |
ATC 2 |
X |
0 |
Level |
None |
ATC |
|
|
ATC3 |
ATC 3 |
X |
0 |
Level |
None |
ATC |
|
|
ATC4 |
ATC 4 |
X |
0 |
Level |
None |
ATC |
|
|
MP |
Medicinal Product Group |
X |
Group Level |
None |
|||
|
PN |
Preferred Name |
X |
10 |
Level |
None |
MP |
|
|
SYN |
Synonym |
X |
0 |
Level |
None |
MP |
|
|
PF |
Pharmaceutical Form |
20 |
Level |
None |
|||
|
STR |
Strength |
30 |
Level |
None |
|||
|
NS |
Name Specifier |
40 |
Level |
None |
|||
|
COUNTRY |
Country |
50 |
Level |
None |
|||
|
MAH |
Marketing Authorization Holder |
60 |
Level |
None |
|||
|
ING |
Ingredient |
70 |
Level |
None |
|||
|
INGSRC |
Ingredient Reference |
0 |
Level |
None |
|||
|
MPSRC |
Reference |
80 |
Level |
None |
|||
|
SRCCOUNTRY |
Source Country |
90 |
Level |
None |
|||
|
COM |
Company |
100 |
Level |
None |
|||
|
PRT |
Product Type |
110 |
Level |
None |
|||
|
PRG |
Product Group |
120 |
Level |
None |
Parent topic: Sample WHODrug Dictionary with Format C or C3
Sample WHODrug Dictionary with Format C or C3 Level Relations Definitions
See Defining Relations Between Levels (Strong Dictionaries Only) for instructions on defining Level Relations in the Define Dictionaries window.
Table A-14 Sample WHODrug Dictionary Format C or C3 Level Relations Definitions
| Setting | Parent Level | Child Level |
|---|---|---|
|
Level Name |
Anatomical-Therapeutic-Chemical- Classification Group |
ATC 1 |
|
Relation Type: Top Sublevel |
||
|
Short Name |
ATC |
ATC1 |
|
Mandatory? |
||
|
Many Cardinality? |
||
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Level Name |
ATC 1 |
ATC 2 |
|
Relation Type: Level |
||
|
Short Name |
ATC1 |
ATC2 |
|
Mandatory? |
X |
|
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
X |
n/a |
|
Level Name |
ATC 2 |
ATC 3 |
|
Relation Type: Level |
||
|
Short Name |
ATC2 |
ATC3 |
|
Mandatory? |
X |
|
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
X |
n/a |
|
Level Name |
ATC 3 |
ATC 4 |
|
Relation Type: Level |
||
|
Short Name |
ATC3 |
ATC4 |
|
Mandatory? |
X |
|
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
X |
n/a |
|
Level Name |
Anat.-Therap.-Chem.-Classification Group |
Preferred Name |
|
Relation Type: Level |
||
|
Short Name |
ATC |
PN |
|
Mandatory? |
X |
|
|
Many Cardinality? |
X |
X |
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
X |
n/a |
|
Level Name |
Preferred Name |
Synonym |
|
Relation Type: Level |
||
|
Short Name |
PN |
SYN |
|
Mandatory? |
X |
|
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Derivable? |
X |
n/a |
|
Level Name |
Medicinal Product Group |
Preferred Name |
|
Relation Type: Level |
||
|
Short Name |
MP |
PN |
|
Mandatory? |
||
|
Many Cardinality? |
||
|
Primary Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Level Name |
Medicinal Product Group |
Pharmaceutical Form |
|
Relation Type: Top Sublevel |
||
|
Short Name |
MP |
PF |
|
Mandatory? |
||
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Level Name |
Medicinal Product Group |
Strength |
|
Relation Type: Level |
||
|
Short Name |
MP |
STR |
|
Mandatory? |
||
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Level Name |
Medicinal Product Group |
Name Specifier |
|
Relation Type: Level |
||
|
Short Name |
MP |
NS |
|
Mandatory? |
||
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Level Name |
Medicinal Product Group |
Country |
|
Relation Type: Level |
||
|
Short Name |
MP |
COUNTRY |
|
Mandatory? |
||
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Level Name |
Medicinal Product Group |
Marketing Authorization Holder |
|
Relation Type: Level |
||
|
Short Name |
MP |
MAH |
|
Mandatory? |
||
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Level Name |
Medicinal Product Group |
Ingredient |
|
Relation Type: Level |
||
|
Short Name |
MP |
ING |
|
Mandatory? |
||
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Level Name |
Ingredient |
Ingredient Reference |
|
Relation Type: Level |
||
|
Short Name |
ING |
INGSRC |
|
Mandatory? |
||
|
Many Cardinality? |
X |
X |
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Level Name |
Medicinal Product Group |
Reference |
|
Relation Type: Level |
||
|
Short Name |
MP |
MPSRC |
|
Mandatory? |
||
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Level Name |
Medicinal Product Group |
Source Country |
|
Relation Type: Level |
||
|
Short Name |
MP |
SRCCOUNTRY |
|
Mandatory? |
||
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Level Name |
Medicinal Product Group |
Company |
|
Relation Type: Level |
||
|
Short Name |
MP |
COM |
|
Mandatory? |
||
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Level Name |
Medicinal Product Group |
Product Type |
|
Relation Type: Level |
||
|
Short Name |
MP |
PRT |
|
Mandatory? |
||
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
|
Level Name |
Medicinal Product Group |
Product Group |
|
Relation Type: Level |
||
|
Short Name |
MP |
PRG |
|
Mandatory? |
||
|
Many Cardinality? |
X |
|
|
Primary Link? |
n/a |
|
|
Primary Path Link? |
n/a |
|
|
Derivable? |
n/a |
|
Parent topic: Sample WHODrug Dictionary with Format C or C3
WHODrug Format C or C3 Data Loaded as TMS Informative Notes
Some WHODrug Format C tables, which are loaded into TMS as dictionary levels, contain too many attributes, or columns, to fit into tables tms_dict_contents and tms_dict_relations. In these cases the sample scripts load certain column data as Informative Notes associated with terms that have data in the column.
All mappings are detailed in the sample script code.
Table A-15 WHODrug C or C3 Data Handled as Informative Notes in TMS
| WHODrug C Table/ TMS Dictionary Level | WHODrug C Table Column | Informative Note Attribute |
|---|---|---|
|
Medicinal Product |
AUTHNUM |
Marketing Authorization Number |
|
Medicinal Product |
AUTHDATE |
Marketing Authorization Date |
|
Medicinal Product |
WITHDATE |
Marketing Auth Withdrawal Date |
|
Medicinal Product and Substance |
REFYEAR |
Year of Reference |
|
Medicinal Product and Ingredient |
CREATEDATE |
Create Date |
|
Medicinal Product |
CHGDATE |
Date Changed |
Parent topic: Sample WHODrug Dictionary with Format C or C3