Site profile shows performance and history
Site profiles store extensive detail about a specific site's contact information, trial history, related facilities, and more.
Note:
Oracle Site Select supports alternate site profiles that can be enabled for your organization upon request. Please contact your Oracle services representative to discuss the site profile that best fits your unique organizational needs.Legacy (default) site profile
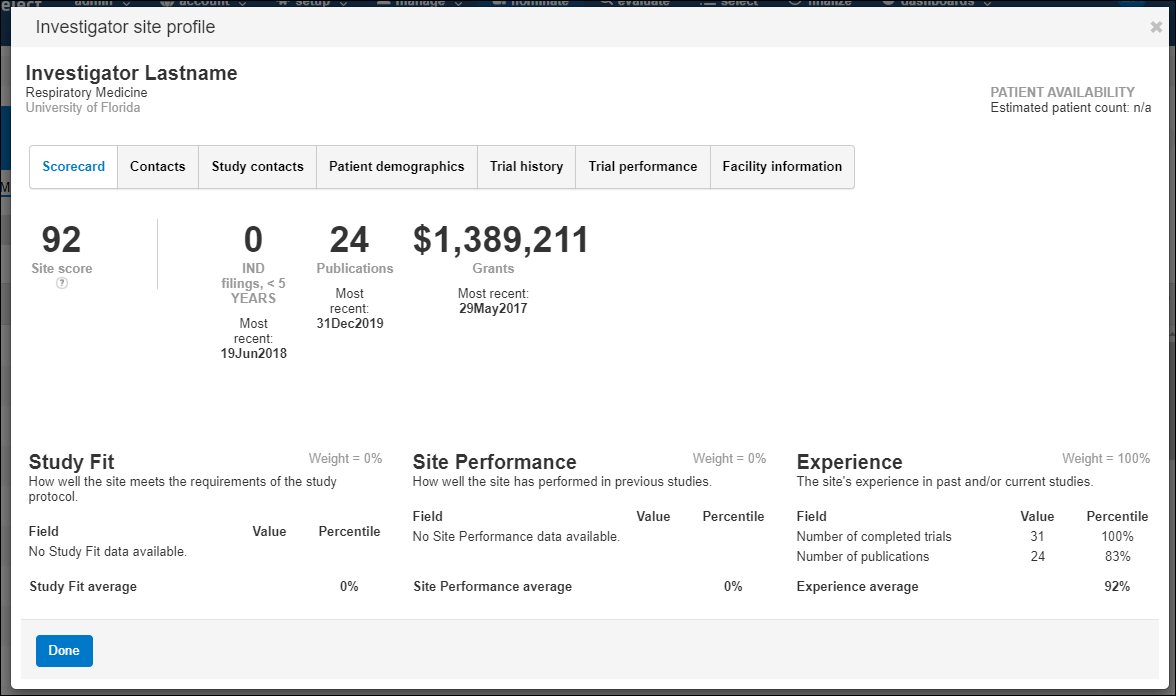
On the Oracle Site Select nominate, evaluate, select or finalize tabs, you can click the site’s profile icon to display a scorecard that shows how the site scored on the study’s key criteria. The scorecard shows the site’s overall score, which is a weighted average of the site’s study fit, performance, and experience.
Oracle Site Select calculates the score using the following formula:
You'll also see the number of site publications and IND filings during the last five years, the value of grants, patient availability, and GCP training. Additionally, you can view the site’s historical score, the scoring algorithm used to calculate the site’s score, and underlying data associated with each site’s score; this provides transparency into the raw data used to generate the score.
Oracle Site Select uses a standard algorithm for all sites to ensure that your site selection process is objective and unbiased. You can export a CSV file of scoring data fields, raw percentile scores, and final weighted score for one or more sites.
Note:
Customers can choose to show or hide various tabs in the site profile; therefore, the images in this section are representative. At the account level, an Oracle administrator can enable/disable display for the following standard site profile tabs:
- Contacts (including the Investigator, Institution, and Site subtabs)
- Contacts > Site (hides only the Site subtab under Contacts)
- Facility information
- Patient demographics
The above site profile tabs display by default, and customers may choose to hide any or all of these tabs as preferred. When hidden, a tab is not visible to site users in Oracle Site Select LITE or to Oracle Site Select users viewing the site scorecard and the Edit site profile page.
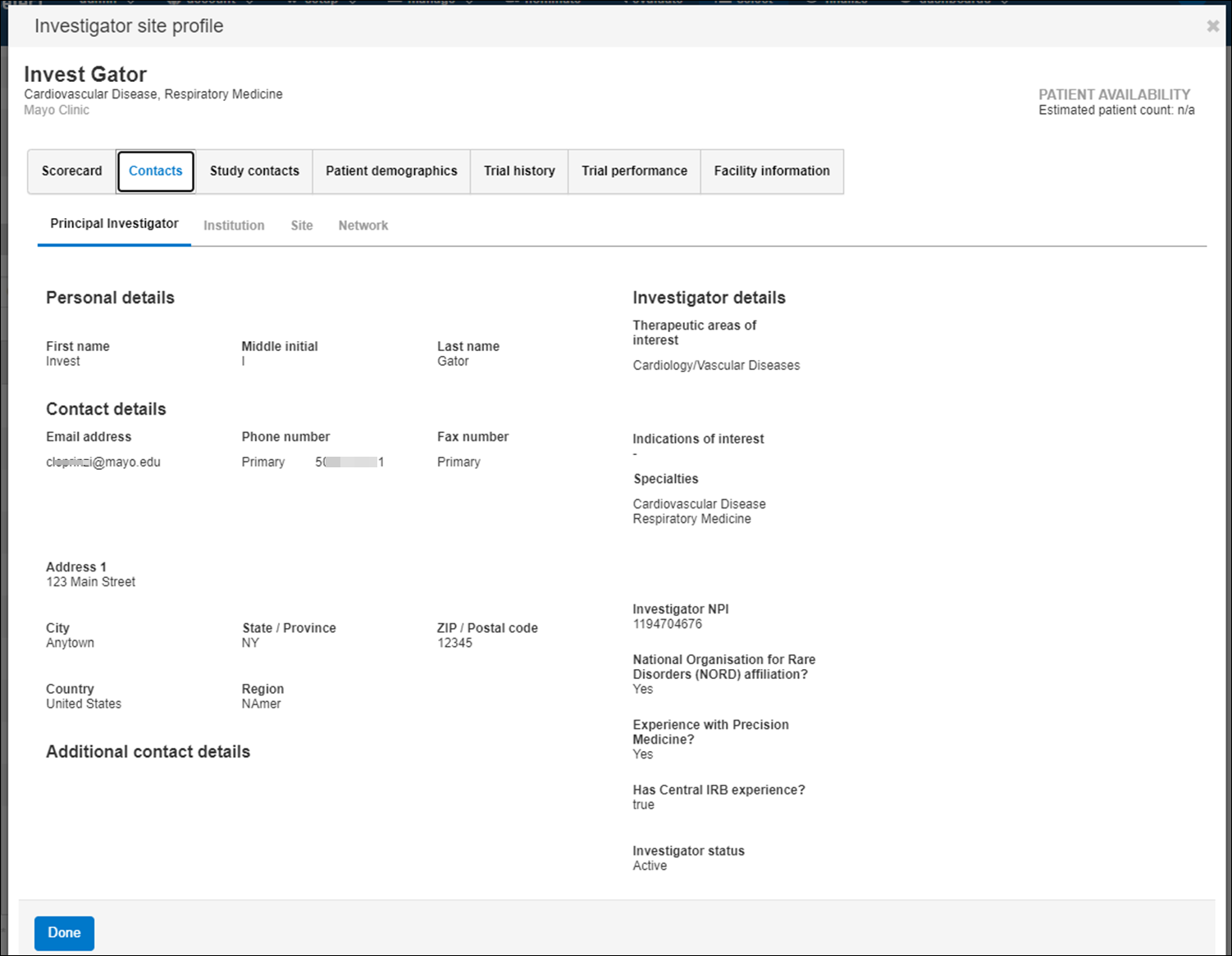
- Contact information for the principal investigator, trial coordinator, IRB coordinator, and other institution staff
- Study contacts (populated with a list of invited site users with listed site users dependent upon email template preferences)
- Regulatory actions (this tab displays only if there is regulatory action data available)
- Patient demographics
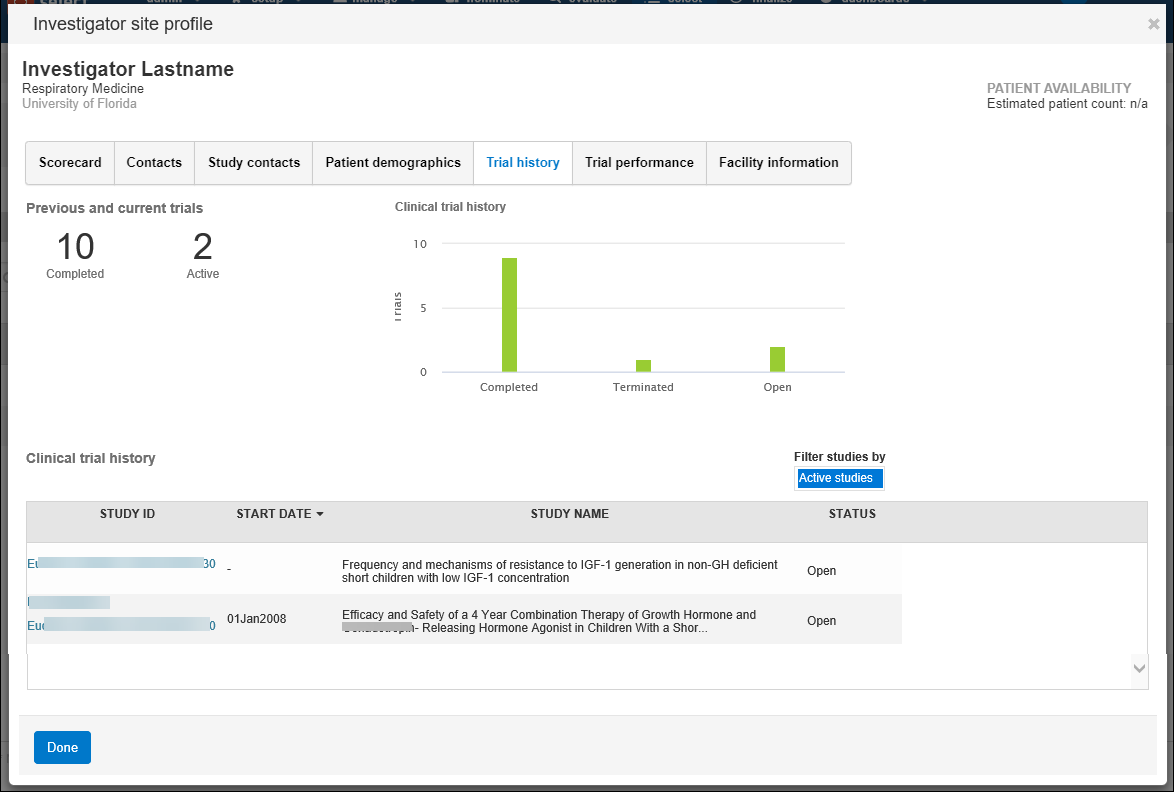
- Trial history (the number and a list of active and completed trials)
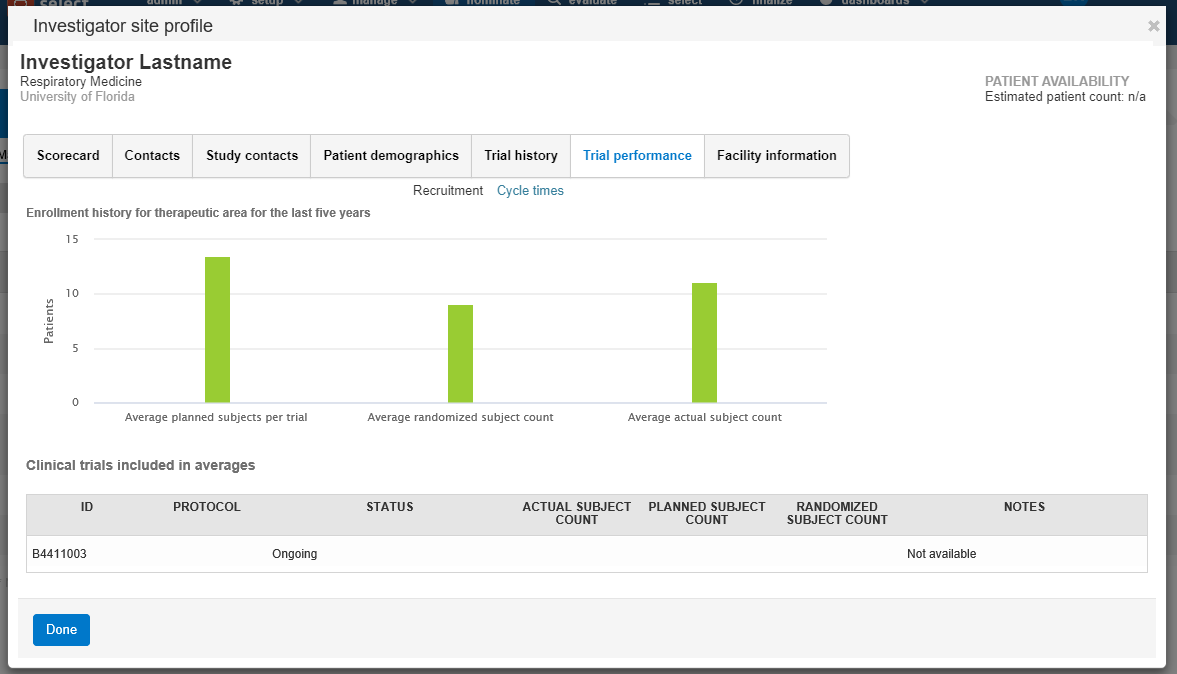
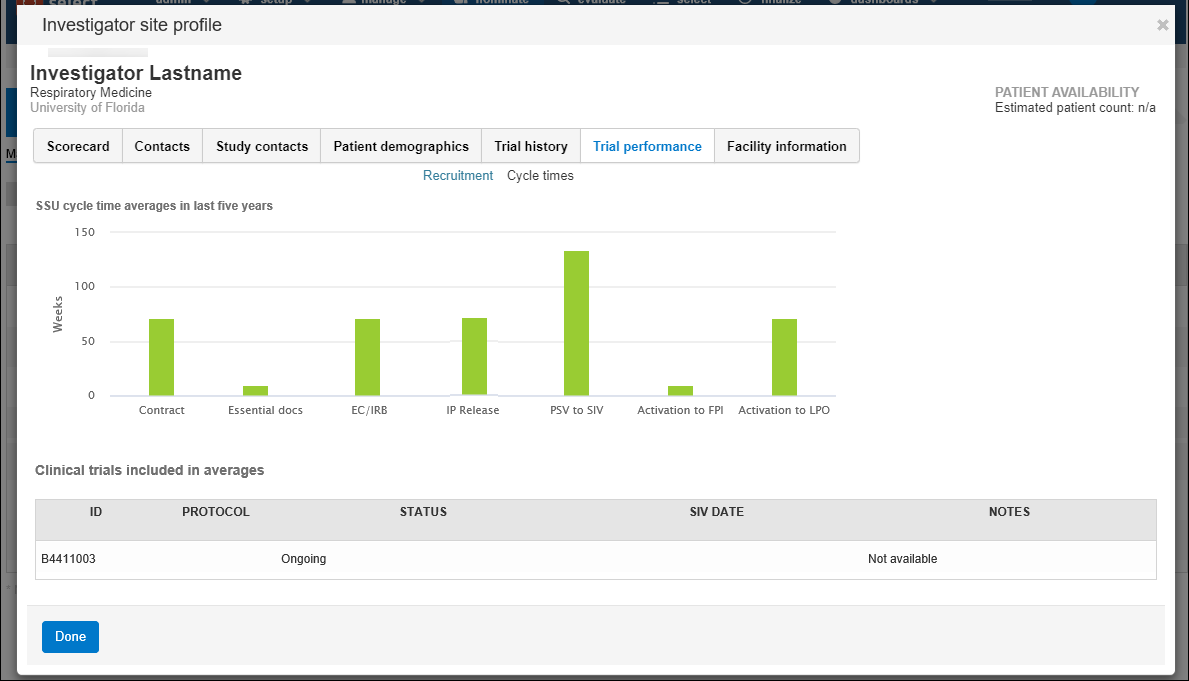
- Trial performance:
- Recruitment – the site’s enrollment history for the study’s therapeutic area over time including the average planned subjects per trial, average randomized subject count, actual subject counts, and a list of studies included in the counts
- Cycle times – the site’s SSU average cycle times for contracts and essential document collection, submissions, IP release, time from prestudy visit (PSV) to site initiation visit (SIV), time from activation to first patient in (FPI), and time from activation to last patient out (LPO)
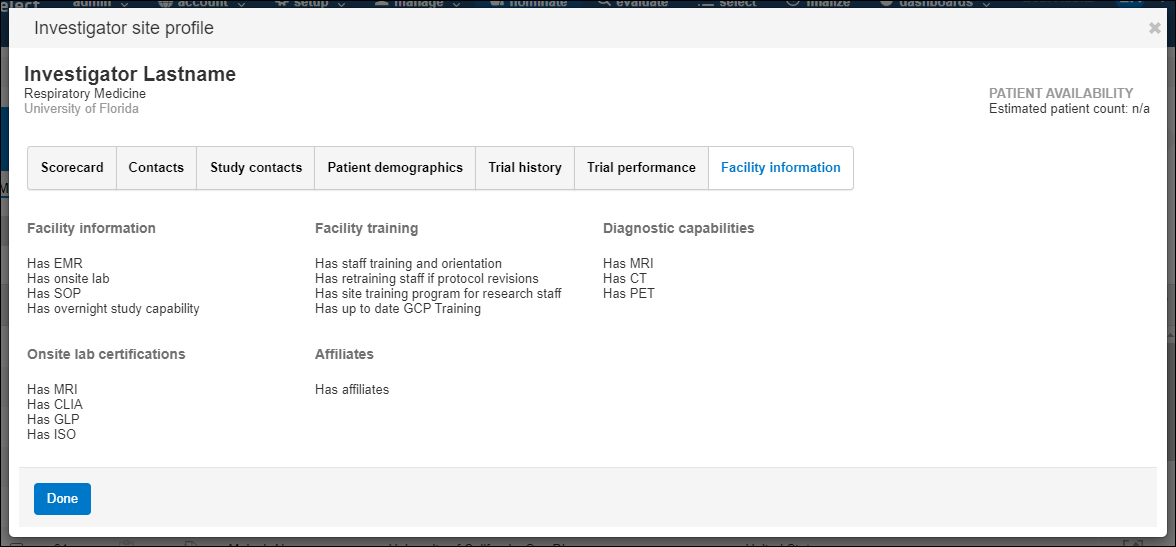
- Facility information – type, diagnostic capabilities, facility information, on site lab certifications, facility training, and affiliates
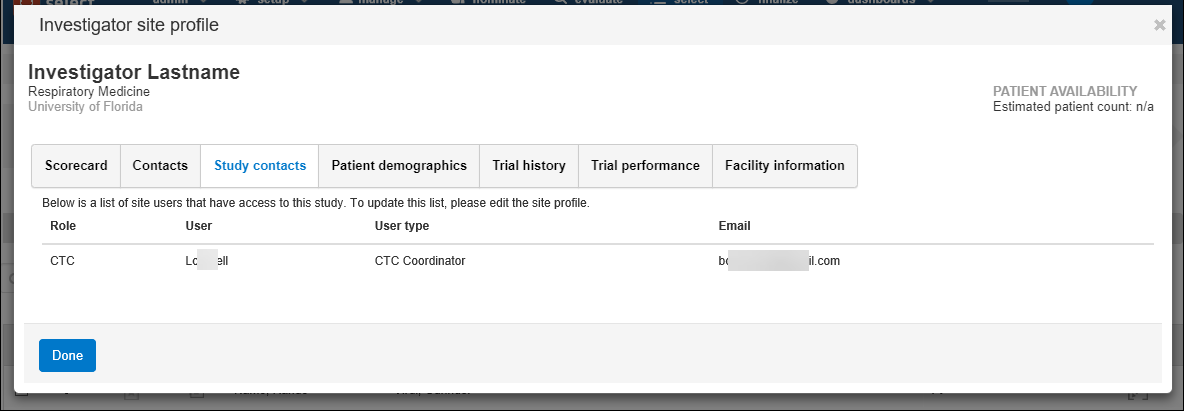
Oracle Site Select and Oracle Site Select LITE users can use the Study contacts tab to identify site contacts and specify, at the study level, which site contacts should have study access and receive notifications. The Study contacts tab displays a grid of invited site users. Oracle Site Select users who have Edit site profiles permission and Oracle Site Select LITE users can add additional contacts, edit assigned roles (CTC or Other), and remove contacts as necessary for any individual.
Study site users listed in the study contacts grid have an assigned role and will receive all future email notifications for the study, as appropriate to the role and study email template settings. Study email template settings affect which users are available to be listed in the study contacts grid and the functionality available for the grid. Below are a few examples of this tab's behavior.
- The PI automatically has the “PI” role.
- The default Clinical Trial Coordinator, defined on the profile’s “Contacts” tab, automatically has the “CTC” role.
- The institution staff users' roles, including an IRB Coordinator defined on the profile’s “Contacts” tab, are listed as “Other.”
- An institution staff user can have their changed from Other to CTC, and the default CTC can have their role changed to Other.
- The “Add” button on the Study contacts tab is disabled because all available users have been invited.
- If the user chooses to Remove a CTC or Other user from the grid, the Add button will be enabled.
- If the user chooses to Add a user to the grid, the only available user will be the user just removed from the grid.
- The PI automatically has the “PI” role.
- “Add” and “Remove” controls on the Study contacts tab are disabled because the read-only PI row is the only role in scope for display on the tab.
- The PI automatically has the “PI” role.
- The default Clinical Trial Coordinator, defined on the profile’s “Contacts” tab, automatically has the “CTC” role.
- The user may not change the default CTC's role, because CTC is the only available option; however, the user can remove the default CTC.
- The user can Add another user (institution staff or default IRB Coordinator) and that staff member will default to the role of CTC, again because CTC is the only available option. This logic allows the study to have multiple CTCs for a study site, if preferred.
- A study invitation email will be sent to any user added to the study preferences as a CTC.
Note:
A contact’s data (title, name, suffix, and degree) displayed in the Study contacts grid reflects the site profile data rather than the data saved in the Oracle Site Select LITE user profile. If a site user changes the Oracle Site Select LITE user profile name, the updates save successfully but do not impact the data displayed on the Study contacts grid. However, when an Oracle Site Select user or a site user updates a study contact site user's name within in the site's profile, the Study contacts grid reflects the updated information.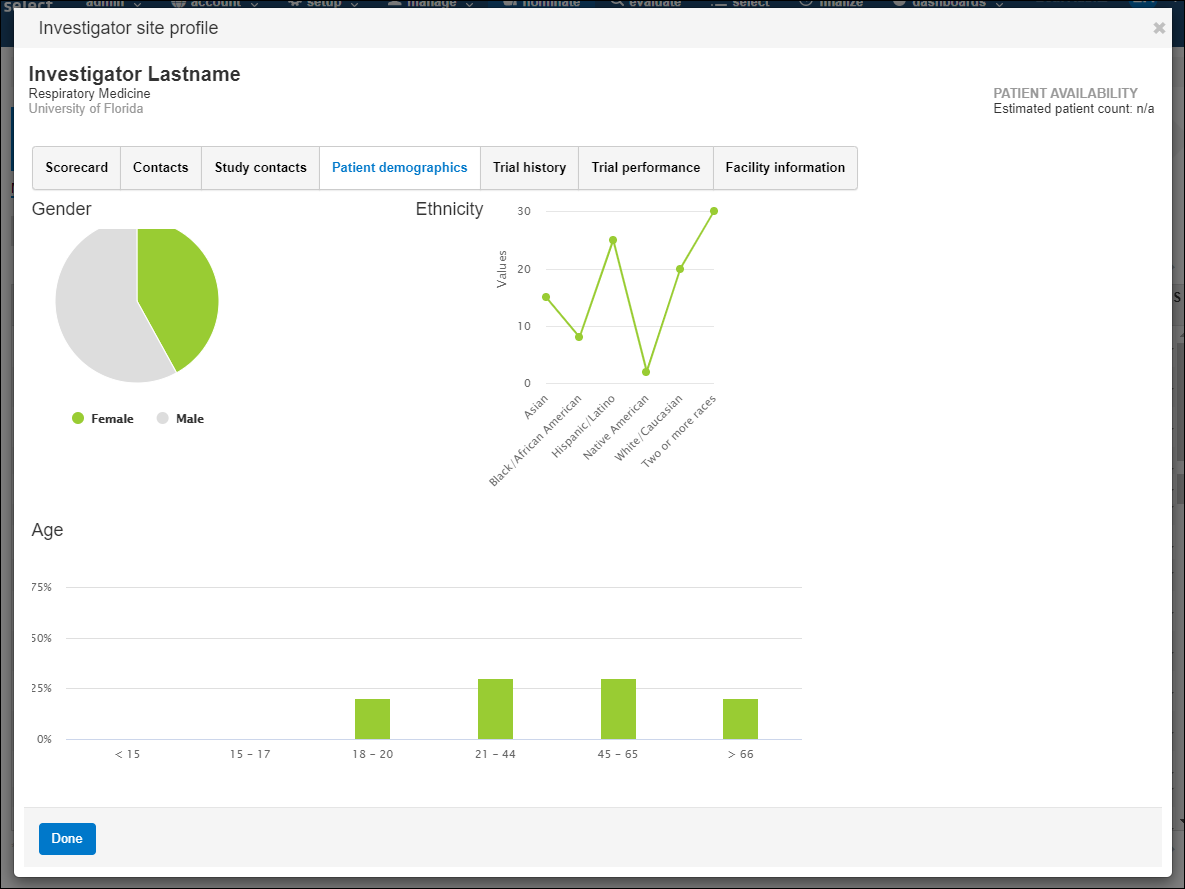
Study-specific site profiles
Oracle Site Select offers a study-specific site profile feature. When enabled for the account by an Oracle administrator, this feature allows site profile updates made within a study to be stored in a data source specific only to that study. This feature is an alternate implementation to the account level site profile (described above). Your organization's Oracle Site Select account will be configured with either account level site profile (default setting) or study-specific site profile.
In Oracle Site Select and Oracle Site Select LITE, profile page titles include the text "for this study only" so users who are viewing or editing the profile quickly see that the profile applies to the current study.
Configurable site profile
Configurable site profile functionality that allows customers to define site profile sections, fields, and field values as required for their unique business needs. Please contact your Oracle administrator for more details on how to map and implement this alternate site profile functionality.
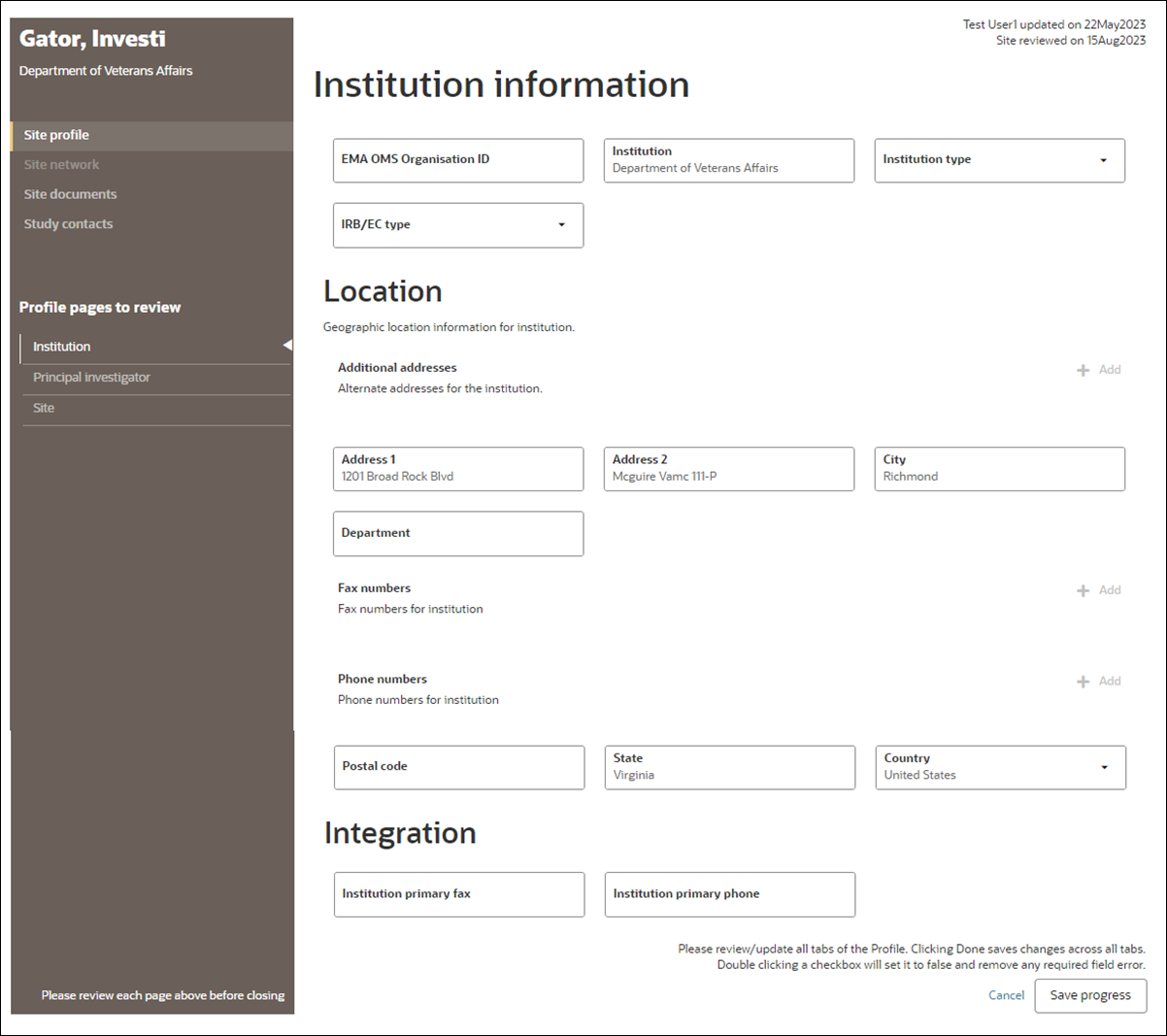
When an Oracle administrator enables configurable site profile functionality for the account, an enhanced design replaces the legacy site profile design (described above). All links and navigation that previously allowed users to access the legacy site profile will access the configurable site profile instead. For example, an Oracle Site Select user completing the site profile task will access the configurable site profile.
In Oracle Site Select, the configurable site profile page design includes section navigation links in a left side panel and uses Oracle standard elements for fields, buttons, and icons. The left navigation also includes links to Site networks, Documents, and Study contacts.
With configurable site profile enabled, all site profile changes are stored in an account-level data source for new studies. Site profile changes will span across all studies in the account that do not currently have a study-specific site profile data source.
Oracle Site Select users must have Edit site profile permission to modify any field in the configurable profile. The profile will be read-only for all other users. This aligns with the required permission for updates to the legacy site profile.
Oracle Site Select LITE will also use the enhanced page design for accounts with configurable site profile enabled.
Parent topic: Site Selection