4 Reports
4.1 Reports: PMDA Expedited Reports
This section describes the J specific PMDA Expedited Report changes.
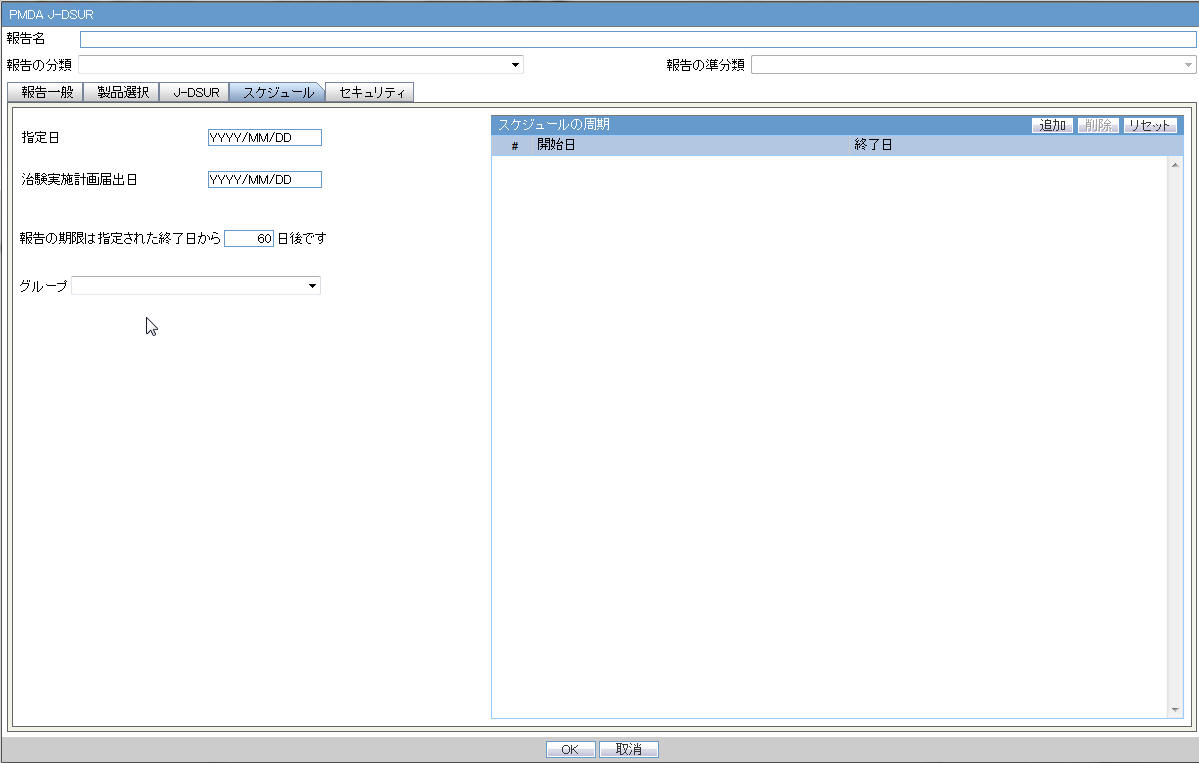
4.1.1 Report Scheduling
As part of the PMDA E2B R3 implementation, the application is enhanced for the reporting related requirements.
-
Urgent Report flag: You can schedule an Urgent report for a Product License based on the value of Case form > Analysis Tab > PMDA Information Tab > General Tab > Urgent Report Checkbox for that License.
-
Aware Date: You can schedule a report for an Aware Date based on the value selected in the Case form > Analysis Tab > PMDA Information Tab > Comments Tab > Start date of reporting timeframe drop-down for that License.
-
Downgrade/Nullification flag: Determines whether the report is Nullification or Downgrade. The preference is given to the manually selected value Case form > Analysis Tab > PMDA Information Tab > Comments Tab > Downgrade Report flag drop-down for that License.
Besides, the logic to schedule Nullification report in case Investigation license for Event invalidation scenarios is updated to be in synchronization with Marketed Report.
-
Nullification Against changed Reporting Category: Enables you to schedule both R2/R3 as Nullification report against a License for which Reporting Category was changed. The new Justification field is used as the Nullification reason.
-
Aware Date/Due Date of a downgrade/Nullification reports: Schedules a Downgrade/Nullification report based on the Aware date and Due Date as per the latest case data rather than copying from previous report.
-
Nullification Report Distribution flag: Enable you to maintain Downgrade/Nullification report status (to not always mark the schedule Downgrade/Nullification report) for Auto Transmission, and follows a regular follow-up report workflow (as configured).
4.1.2 Paper Reports
Argus J is able to generate and submit the following expedited paper report formats specified by PMDA (both E2B R2 and E2B R3) for drugs.
PMDA forms for Marketed Drugs:
-
Drug AE/Infection case report form 1
-
Drug AE/Infection case form 2 (5pages)
-
Surveillance report on drug, quasi drug, cosmetic case report form 3
-
Surveillance report on drug, quasi drug, cosmetic case report form 4
-
Surveillance report on measures taken for drug outside Japan such as production termination, recall, rejection, etc. form 5
-
Surveillance report on measures taken for drug outside Japan such as production termination, recall, rejection, etc. form 6
PMDA forms for Investigational Drugs:
-
Investigational product AE/Infection case report form 1
-
Investigational product AE/Infection case form 2
-
Surveillance report on investigational product research report form 3
-
Surveillance report on investigational product research report form 4
-
Surveillance report on measures taken for investigational product outside Japan such as production termination, recall, rejection, etc. form 5
-
Surveillance report on measures taken for investigational product outside Japan such as production termination, recall, rejection, etc. form 6
Besides, for PMDA R2 Report—Paper Report generation logic for PMDA Marketed Form 1 and 2, and PMDA Investigation Form 1 and 2 are enhanced to be in synchronization with CIOMS and MedWatch reports for different type of users.
The Report Form List is used in the following sections:
-
Console J - Expedited Report Rules - Form section (irrespective of whether Japanese module is enabled or disabled)
-
Schedule New Expedited Report dialogue - Form section (only if Japanese module is enabled)
-
Medical Review - Preview of Expedited Report (only if Japanese module is enabled)
If an initial paper report (or a follow-up) was scheduled with Agency having the PMDA E2B R2 profile, and now the Agency configuration is updated to E2B R3 profile then the paper report respects the ongoing series and a new follow-up is generated in R3 paper format.
Note that, when submitting a follow-up report for a report submitted and received as an R2 Report, it is possible to switch to an R3 Report. However, submitting a follow-up report for a report submitted and received as an R3 Report, you will not be able to switch to an R2 Report.
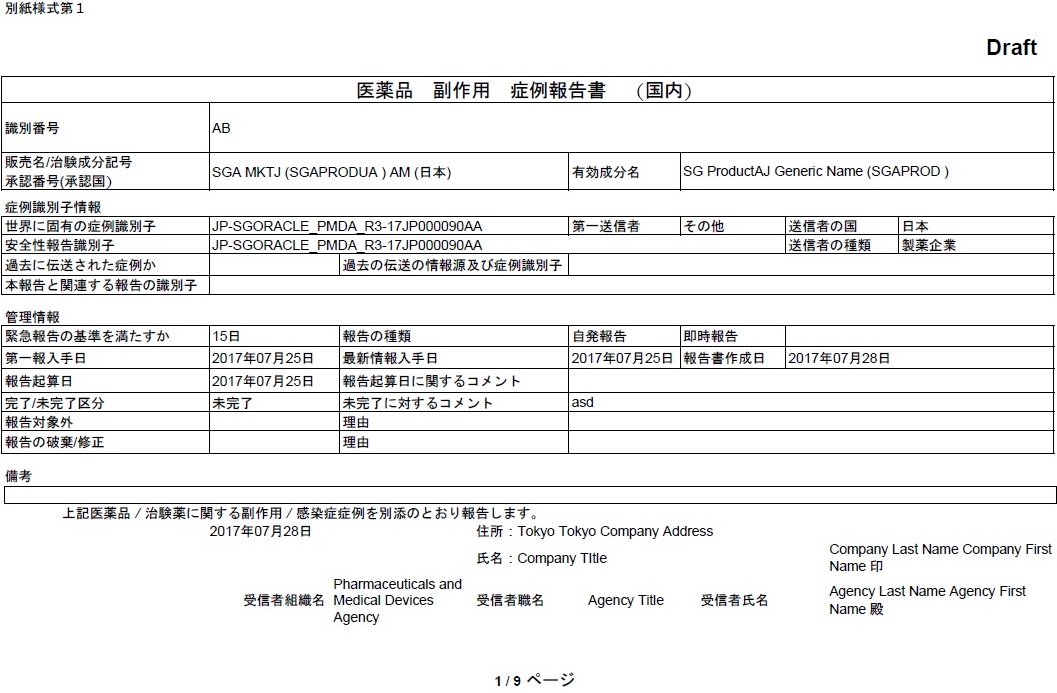
Sending a FAX report (PMDA Paper Report Form) for immediate notifications
In case of a death in a case, it is now possible to generate and send a FAX report for immediate notification to PMDA.
In the PMDA E2B profile, the Argus Safety application distinguishes the PMDA reporting destination from other partner reporting destinations configured in Argus Console, based on the Agency Type and Agency Country fields configured in the Reporting Destination code list.
The E2B or Paper Report is scheduled for PMDA only when the When Agency Type is Regulatory Authority and Country is selected as Japan.
In the event of this not being the case, the application will not transmit the J.4b value in it and will also not perform the associated validation for the J.4b value.
The E2B Viewer > PMDA Paper Report Forms view still displays the associated J.4b value for PMDA E2Bs as it is still based on an E2B report.
4.1.2.1 Additional Expedited Report Updates
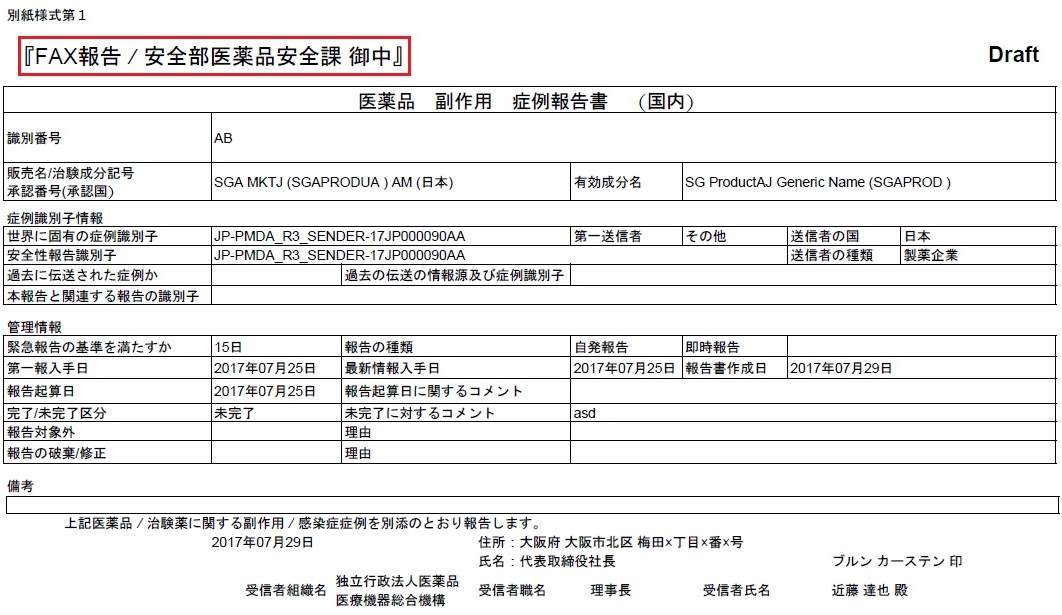
The Expedited reports which are faxed to the PMDA need to be identified by which receiver is sending the information. This is done by the Additional Header information on the report output.
-
The following profile switches are added to Console > System Configuration > Argus J > Reporting. These switches are specific to the Enterprise:
# Field Label Field Length 1 Marketed: Form 1,2 40 2 Marketed: Form 3,4 40 3 Marketed: Form 5,6 40 4 Investigational: Form 1,2 40 5 Investigational: Form 3,4 40 6 Investigational: Form 5,6 40 These switches are depicted in the following figure:
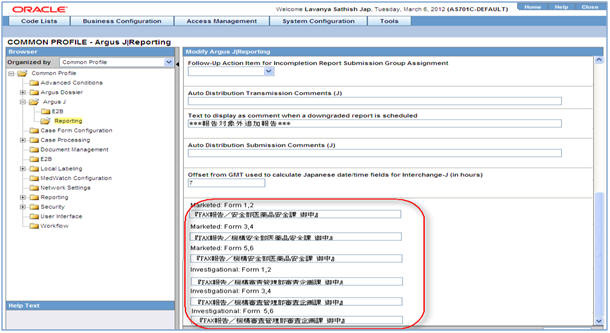
-
The Reporting Destination Configuration has an Include FAX Header on PMDA Paper Reports Argus J user-specific checkbox on the Agency Configuration tab.
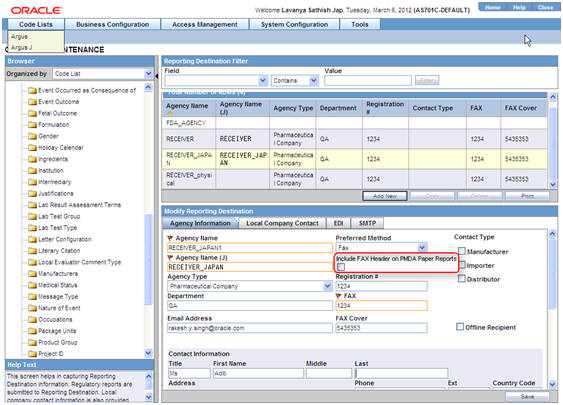
-
This checkbox is enabled only when FAX is selected as the Preferred Method and is reset to disabled and blank when Preferred Method is set to something else.
-
By default, this field is unchecked for existing and newly created Reporting Destinations.
-
You can mark this option for a Reporting Destination and the changes made to this flag are audit logged and printed as part of Codelist Maintenance Print PDF.
-
Fax header is printed only if the Include FAX Header on PMDA Paper Reports is marked for that Reporting Destination.
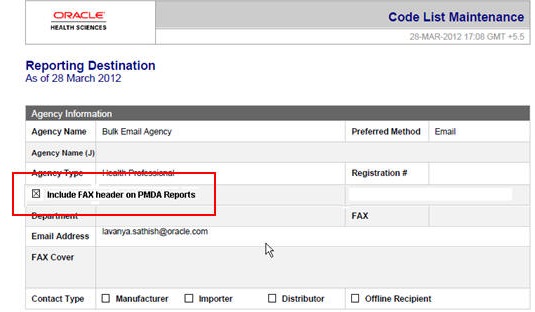
-
Text entered in the Common Profile Switch is printed on the first page of the PMDA Paper Report:
-
Fax header text is printed in MS Mincho font of size 16.
-
Fax header text is printed in the line below the Report Format Number and is aligned to the left.
-
If you clear the Fax Header Text for the PMDA Paper reports in the Common Profile Switch, the header is left blank in the report.
Note printed on PMDA Paper Reports > Marketed Forms 1, 3 and 5 is removed as it is a data entry description (for FAX as well as when sent through Email ) Report Note to be removed (English Translation) Marketed Form 1 Note: Format No.2(1) to (5) have to be attached Marketed Form 3 Note: Form 4 should be attached together. Marketed Form 5 Note: Attach Form 6 together. The following figure depicts the PMDA Paper Report Sample:

-
-
PMDA Marketed Form 1 and 2, and PMDA Investigation Form 1 and 2 Paper Report generation logic are enhanced to be in synchronization with CIOMS and MedWatch reports for different type of users.
User Description Restricted User for whom Console > Access Management > Argus > User > "Protect from Printing Unblinded Information" is checked. Non-Restricted User User for whom Console > Access Management > Argus > User > "Protect from Printing Unblinded Information" is not checked. Blinded Study Case Case with a blinded study which is still not eligible for unblinding irrespective of the fact that the blind for the case is broken or not it shall be considered as a blinded case. Blinded Report The report for which the configured Blind Study Product is marked as Yes. -
When a restricted user generates/prints the report for a Blinded Study Case (Blinded report and Not Blinded report), if the "Blind in PMDA AE Paper Report" checkbox is selected, the profile elements are printed as blinded.
-
When a Non-Restricted user generates:
Blinded Report: The application generates/prints a report as a Blinded Study Case, if the "Blind in PMDA AE Paper Report" checkbox is selected, and the profile elements are listed as blinded.
Not Blinded Report: A "Do you want to hide Unblinded Information?" dialog box appears.
If you select "No", the report is printed without blinding the any information.
If you select "Yes", the report is printed after blinding the profile elements for which "Blind in PMDA AE Paper Report" checkbox is selected.
-
For blinding B.4.K.2.1 MEDICINALPRODUCT and B.4.k.2.2 ACTIVESUBSTANCENAME, the report is printed in the following format:
B_<Blinded Study Name>(B_<Study Level CCN)>)
-
Blinded G.k.2.2 & G.k.2.3.r.1/ G.k.2.3.r.2(a,b)/ G.k.2.3.r.3(a,b) are printed as:
-
G.k.2.2 MEDICINALPRODUCT—Prints <Study Level CCN> for the PMDA Paper report forms 1-2.
For blinded output, when CCN from J2.12 tag is printed for G.k.2.2, J2.12 is suppressed, so that it is not repeated in the same field.
-
G.k.2.3.r.1/ G.k.2.3.r.2(a,b)/ G.k.2.3.r.3(a,b) ACTIVESUBSTANCENAME—Prints <Generic Name from Case form> for the PMDA Paper report forms 1-2.
In case G.k.2.3.r.1 is required to be printed along with G.k.2.3.r.2 or G.k.2.3.r.3, for blinded output, the application prints <Generic Name from Case form> only once without repeating in place of G.k.2.3.r.2 or G.k.2.3.r.3.
-
For blinded output, products that are only study drugs are blinded. All non-study drugs in the case are not blinded in the Paper Form.
-
-
Blinding J.10/J2.11 (mhlwadmicsrreporttimesevent) element is governed based on the "Blind in PMDA AE Paper Report" in ESM Mapping and "Blind J.10/J2.11 in PMDA AE Paper" of the License for which the report is scheduled (PMDA E2B R2 or PMDA E2B R3 respectively).
a. If in PMDA Profile, for J.10/J2.11 "Blind in PMDA AE Paper Report" is unchecked, then irrespective of the value the checkbox "Blind J.10/J2.11 in PMDA AE Paper " for the corresponding License against which the report (blinded or not blinded) is scheduled, and also irrespective of the Blinded status of the case, J.10/J2.11 is not be blinded for both Restricted as well as Non-Restricted user.
b. If in PMDA Profile, for J.10/J2.11 "Blind in PMDA AE Paper Report" is checked:
i. If the checkbox "Blind J.10/J2.11 in PMDA AE Paper " " for the corresponding License against which the report is scheduled is checked then irrespective of the Blinded status of the case when a restricted user prints/views the PMDA Paper Report Form 1-2 MKT/INV the application always Blinds the J.10/J2.11 data in the report, and when Non-Restricted user prints/views the PMDA Paper Report Form 1-2 MKT/INV:
* Blinded Report: The application prints/views the PMDA Paper Report Form 1-2 MKT/INV, and always Blind the J.10/J2.11 data in the report.
* Not Blinded report: The application prompts a "Do you want to hide Unblinded Information?" dialog box. If you select "No", then the report is printed without blinding the J.10/J2.11, but if select "Yes", then the report is printed after blinding the J.10/J2.11.
ii. If the checkbox "Blind J.10/J2.11 in PMDA AE Paper " " for the corresponding License against which the report is scheduled is un-checked, and the case is a blinded study case, when a restricted user prints/views the PMDA Paper Report Form 1-2 MKT/INV the application Blinds the J.10/J2.11 data in the report, and when Non-Restricted user prints/views he PMDA Paper Report Form 1-2 MKT/INV:
* Blinded Report: The application prints/views the PMDA Paper Report Form 1-2 MKT/INV, and always Blinds the J.10/J2.11 data in the report.
* Not Blinded report: he application prompts a "Do you want to hide Unblinded Information?" dialog box. If you select "No", then the report prints without blinding the J.10/J2.11, but if you select "Yes", then the report prints after blinding the J.10/J2.11.
iii. If the checkbox "Blind J.10/J2.11 in PMDA AE Paper" for the corresponding License against which the report is scheduled is un-checked and the Case is not a blinded study case, irrespective of the restricted and Non-Restricted user J.10/J2.11 is not blinded.
-
You can now print the submitted Blinded PMDA Paper Report from Reports > Compliance > Submitted Reports using Print Submitted Report without getting the following error:
"Cannot print PMDA Paper Report for J E2B (Report Form Id = <report form id>, Case Num = <case num>) as user does not have access to blinded information."
-
4.1.2.2 Printed Paper Reports—Formatting Details
The general guidelines while printing the data in the paper form:
-
Prints textual values instead of the coded values to aid human readability.
-
If the number of rows to be printed in a multi-record section does not fit a page, then the same section is continued to the next page along with the table header until all the records are printed completely.
-
If a non-repeatable field value exceeds the space available in the page, it is continued to the next page to print the overflowing value (like in MS word) until the full value is printed completely. All other fields are printed as per the format.
-
The separators are used as:
-
Comma ','—Multi byte
-
Bracket '(' and ')'—Multi byte
-
Colon ':'—Multi Byte
-
Dash '-'—Single byte
-
-
Null flavor printing is also supported in Paper Forms.
-
True/False is printed in all forms as Yes/No respectively.
-
A form starts only in a new page in the PDF irrespective of previous page data.
For example, if Form 2 Page 1 data is printed from page 3 of a report till page 4, then Form 3 Page 2 data is printed starting from page 5 and not from page 4 itself.
-
If the "Include FAX Header on PMDA Paper Reports" checkbox under the Reporting Destination > Agency Information tab is checked, then the Fax header is printed in the report as configured in the common profile switches for the respective PMDA paper report under Console > System Configuration > Argus J > Reporting.
-
Text entered in Common Profile Switch is printed on the first page of the PMDA Paper Report.
-
Fax header text is printed in MS Mincho with font size 16.
-
Fax header text is printed in the line below the Report Format Number and is aligned to the left.
-
-
The page number is printed in the Footer (center aligned) for each page.
Format: <Current Page Number>/<Total Number of pages>
-
The report page number for each page (other than the main page 1, 3, and 5) is printed in the first table within the label
 .
.Format: Report page number<space>/<space>Total pages in that report
4.1.3 User Interface - Reporting Rules Configuration
This functionality allows configuration of Regulatory Reporting rules for scheduling Japanese reports.
The following are the functionality changes related to the PMDA Expedited reports:
-
The License Category and Reporting Category fields are visible in Audit Log for Expedited Reporting Rules.
-
The Reporting Rules algorithm has been updated to respect the License Category configured in Expedited Reporting Rules.
-
The Reporting Category and License Category fields are printed below the Cover Letter field label under the Expedited Report Rule Information section of the print output.
-
If a report is generated for a License with PMDA Device Classification 1 configured either

Description of the illustration ''fig6.jpg''
then a warning Incorrect PMDA Device Classification 1 configured for the license is displayed. On continuing, the report will display no information for PMDA Device Classification 1.
4.1.4 Expedited Reports on DLP
In addition to supporting revisions supported by local data entry and local locks, DLP has been enhanced to address existing gaps faced by customers while generating Expedited Reports in DLP.
A version in DLP is defined as case data that corresponds to an aware date when a significant data was received (initial receipt date or significant follow up dates).
A revision is defined as committed case data changes that spans from one version to the other and that always corresponds to a locked version.
When DLP is enabled for Expedited Report Generation:
-
Global Expedited Reports are generated based on the latest DLP case revision that corresponds to the aware date on which the report was scheduled. Global reports has been generated even if the case is in unlocked state if a case version exists for that aware date on which the report was scheduled. This is applicable to both Draft and Final reports.
-
If a lock does not exist for the Aware Date on which the report was scheduled, the application picks up the next immediate DLP case revision for a later Aware Date for which a lock exists.
-
If no lock exists for any later aware date, see below.
Note that this is an outlier condition and the customer is expected to lock the revisions for which reporting obligation exists.
If no lock exists at all for any case revision, the report will not be generated, until the case is locked. Similarly, the Case Form will not display the Final report hyperlink or the Regenerate Report menu.
Further, the Batch Report Generation AG Service will not process the case. However, if the report is run as Draft, the report generates the draft, based on the latest case revision, even if no lock exists for any case revision.
Till this release, if more than one aware date existed in between case locks (global), the reporting scheduling algorithm determined the due date of the report based on the latest aware date, instead of the earlier aware dates in the same case processing cycle.
This has now been corrected so that the report scheduling algorithm determines the due date of a report against the earliest aware date (i.e., significant follow-up) in the case for the current case lock cycle.
Note that in this scenario, the reported data is based on the case revision corresponding to the later aware date. Also note that this scenario occurs mostly when the "Case Form Configuration | Auto Regulatory Scheduling" option is set to "Significant" or "Manual".
-
Local Expedited Reports are generated based on the latest DLP local case revision corresponding to which a local lock exists for Japan country for the Japanese aware date on which the report was scheduled (irrespective of current local lock state). This is applicable to both Draft and Final reports.
-
Note that the Japanese aware date is the J initial received or J significant follow-up dates if the USE_JPN_AWARE_DATE is enabled for the Reporting Destination. If the switch is not enabled, it is the global aware date. This is existing functionality.
-
If a local lock does not exist for the aware date on which the report was scheduled, the system shall pick up the next immediate DLP local case revision for a later aware date for which a local lock does exists. If no local lock exists at all for any case revision, the local report shall not be generated. However, if the report was run as Draft, the report shall generate the draft based on the latest case revision even if no lock exists for any case revision, or for any case revision for an aware date later to the aware date for which the report was scheduled.
-
J Expedited Reports always considers the J initial received and J significant follow-up dates as J aware date for determining due dates if the USE_JPN_AWARE_DATE is enabled for the Reporting Destination as with the existing functionality. If there is no Japan aware date or if the USE_JPN_AWARE_DATE is not set, the system defaults to the global aware date. This is applicable during follow-ups as well i.e., when there is no corresponding J follow-up date for a follow-up, the system takes the default global follow-up date.
4.1.5 PMDA Device Reports
-
Two new Japanese expedited device report forms have been added to all expedited report listing sections in Argus Safety.
-
The following form options are displayed (in Japanese) to both English as well as Japanese users:
Report Form 8: Medical Device Malfunction/Infection Case Report (Form 8)
Report Form 10: Medical Device Research Report/Measure in foreign countries Report
-
These form options are displayed to both Japanese as well as English users.
-
These form options are supported at all the following locations in Argus Safety:
Console J - Expedited Report Rules - Form Section (irrespective of whether Japanese module is enabled or disabled)
Console J - Code list / Batch report generation (irrespective of whether Japanese module is enabled or disabled)
Case Form - Toolbar - Draft (only if Japanese module is enabled)
Schedule New Expedited Report dialogue - "Form" dropdown (only if Japanese module is enabled)
Medical Review - Preview of Expedited Report (only if Japanese module is enabled)
Create Unscheduled report (only if Japanese module is enabled)
View Submitted report (irrespective of whether Japanese module is enabled or disabled)
Bulk Report by Form (irrespective of whether Japanese module is enabled or disabled)
-
These forms are not added to the Utility Blank Report Form User Interface as the Utility Blank Report Form feature has been deprecated from Argus Safety 7.0.1 release onwards.
-
-
These report forms open up in Microsoft Word format (.doc) from all the places in Argus Safety application as specified (but not restricted to) in the above specified list using the following forms provided by PMDA retaining all its fields, dropdowns and macros. However, these WORD forms are not meant to be edited and updated back into Argus Safety application.
-
The message box which prompts to ask the user if the expedited report will print blinded data or not is not displayed for these Japan Device Report Forms as there is no blinded data printed in these reports.
-
During Bulk Transmit Email process, these WORD report forms will not be merged even if Reporting Destination - Report Transmission Options have been configured to do so.
Note:
Merge feature is not required for these reports because the frequency of these Japan Device reports is quite low and there will hardly be any point of time when multiple WORD forms would exists in the system pending to be transmitted to a reporting destination.
-
-
These report forms are generated in Microsoft Word format (.doc) itself from both Draft and Final mode.
Note:
These Japan Device Report Form 8 and 10 are not required to be supported on DLP case revision data as of now, because other PMDA Paper Reports and E2B are also not supported on DLP case revision data. -
No mandatory field validation will be done during generation of these report forms, except for the following. All other validations are expected to be achieved by using Case Form Field Validations feature on the desired fields of the PMDA Device Information section.
-
If the value specified in Case form/Products tab/Device sub tab/PMDA Device Information section/Medical Device Reporting Category dropdown for the product for which the report is scheduled does not match with the allowed values for Form 8 or Form 10, then instead of opening the Report Form, application displays an error in the standard Argus Safety message box with OK button and the following message:
'Case does not have matching device reporting category for the selected device report form'
-
The same error is logged as a report generation error, including if it occurs during background generation through Argus Safety Service. This error is also visible in the Report Details dialog - General tab at the bottom, as is displayed for the E2B generation errors.
-
-
Regulatory Reporting Rules Algorithm:
-
The Argus Safety Expedited Reporting Rules engine also considers "Device Reporting Category" dropdown value while matching rules against cases. It gets compared against the Case Form ' Products tab ' Device sub tab ' PMDA Device Information section ' Medical Device Reporting Category dropdown field value.
-
Argus Safety Expedited Reporting Rules engine scheduled the due date for these Japanese Device reports based on the Japan Aware Date, as configured in Reporting Destination configuration.
-
The Argus Safety Expedited Reporting Rules engine schedules only one Japanese Device Report for each matching rule, for each suspect device product in a case, even if the case has multiple Japanese Device Licenses of the same License Type in the Event Assessment tab of the case. It also respects the Reporting Destination configuration for "Suppress Duplicate Reports", as configured.
-
If multiple matching product licenses of the same device license type exist for a suspect device product in the Event Assessment tab, the license with the earliest award date of the matching product is considered for scheduling the report.
-
If the original product license for which the initial / previous follow-up report was scheduled is no longer available but another product license of the same product matching the same rule exists, then a follow-up report is scheduled in subsequent cycles instead of a downgrade.
Downgrade is scheduled only when no licenses for that product matching that rule exist in the case.
-
-
Draft report view: The following logic is followed for PMDA Device Report Forms 8 and 10 to identify the product license when these reports are opened up in draft view, where no product license and reporting destination is not specified:
-
The Draft report view is applicable to the following areas:
Toolbar - Draft icon
Medical Review screen
-
It uses the first matching suspect device product as per the sort order of products in the case form products tab which has the matching "Medical Device Reporting Category" specified in the "PMDA Device Information" section.
The following criteria is used to find matching Medical Device Reporting Category for the Japanese Device Form:
Allowed Device Reporting Categories for PMDA Device Form 8:
a. Malfunction without health damage
b. Malfunction with health damage
c. Infection
Allowed Device Reporting Categories for PMDA Device Form 10:
a. Research Report
b. Measures in Foreign Country Report
Earliest award date: Japanese marketed device active license (withdrawn date later than the Initial Receipt date) of such product is used.
If Japanese marketed device license is not available, the Japanese investigational device license matching the above specified criteria is used.
If no such Japanese device license is available for the matching product, then it considers matching any (irrespective of license country or license type) earliest award date active device license for the product.
In case of multiple device licenses with same award date, it uses the license with the earliest internal license id (primary key) value.
-
If no product exists with the matching "Medical Device Reporting Category" for the Report Form, then instead of opening this form, the application displays an error in the standard Argus Safety message box with OK button and message - "Case does not have matching device reporting category for the selected device report form".
-
It uses the agency as specified in the Argus J common profile switch - "Default name of Regulatory Agency for Draft Expedited PMDA Reports" for such draft report views.
-
-
Manual Report Scheduling dialog for Argus J users is modified as specified below:
If the user selects "Downgrade Report" as a dropdown option in the dialog header, then the "Report Form" dropdown list is updated to only display E2B and PMDA Marketed and Investigational Forms 1 - 6. Other expedited report forms or PMDA Device Report Forms are not displayed for "Downgrade Report" option.
4.2 Reports: PMDA Periodic Reports
In Japan, the PMDA accepts CSV format of Non-serious, Unlisted Periodic Report as well as paper format, and many pharma companies have started to submit using CSV forms. The following is the list of the functionality changes as a result of this requirement:
-
PSR ReSD configuration is enhanced by adding parameter for generating Non serious, Unlisted PSR in Paper and CSV format.

Configuration Parameter Validation Rule Radio button with options Paper Report(FD Report) When TBD is set to Paper Report in the PSR Configuration screen, Non-serious, Unlisted PSR(Form 7-1 and 7-2) are generated in the Paper Form.When TBD is set to FD Report in the PSR Configuration screen:The Form 7-1 and Form 7-2 checkboxes in the PSR Configuration screen are checked and disabled.The Print the report content as Case Listing in the separate paper PSR Configuration parameter is unchecked and disabled.Non-serious, Unlisted PSR (Form 7-1 and 7-2) are generated in CSV format.The Form 7-1 is generated in Paper format along with other PSR/ ReSD forms. -
The radio button is set to Paper Report in the existing PSR Configuration for an upgraded database, if Form 7-1 or Form 7-2 is marked.
-
The Argus Safety standard Advanced Conditions control (as present in English Periodic Reports) is provided in PSR ReSD Configuration screen for inclusion or exclusion of cases for all forms of PSR and ReSD reports.

UI Element Validation Rule Dropdown The drop-down list displays the default options: None and New in the drop-down list. None is displayed by default.The Advanced Condition names are displayed in the alphabetical order.On specifying an Advanced condition, the system runs the query on the cases retrieved from Product selection and Reporting Time frame. Advanced Condition Lookup button The Advanced Condition Lookup dialog box is displayed on clicking this button. AC On clicking the AC button, the system displays a message with Yes, No, and Cancel buttons.If you select Yes , the system displays the Advanced Condition Query Set screen.If you select No, the system displays the Advanced Condition screen.If you select Cancel, the system closes the message.If you do not have rights to create or modify AC's, the AC button on the Japanese Periodic Report Configuration screen displays the following message in Japanese: You do not have the rights to create new Advanced Conditions.If there is no AC specified in Japanese Periodic Configuration screen, the Advanced condition (None) is printed.If there is an AC specified in Japanese Periodic Configuration screen, the Advanced condition(<AC Name>) is printed..
-
The Data population logic of FD report is the same as that of the Paper Report.
-
Every J Periodic Report or Form (not limited to the ones specified below) that fetches data based on the E2B Report submitted to the authority also considers the PMDA E2B R3 report along with the PMDA E2B R2 report.
-
PSR (Form 3, and 4)
-
ReSD (Form 4,5,7,8, and 9, and Listed/Unlisted Tabulation)
-
Seiyakukyo Clinical and Domestic Study Cases
-
4.2.1 DLP at Report Level
-
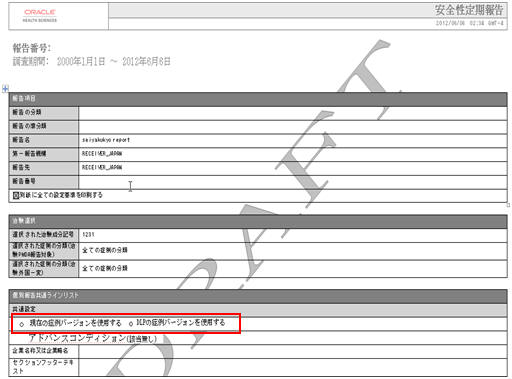
The DLP Configuration radio buttons, Use Current Case Version and Use DLP Case Version is moved to the Report level for PSR/ReSD, CTPR, and Seiyakukyo reports. The PSR/ReSD Report Configuration screen is shown below:
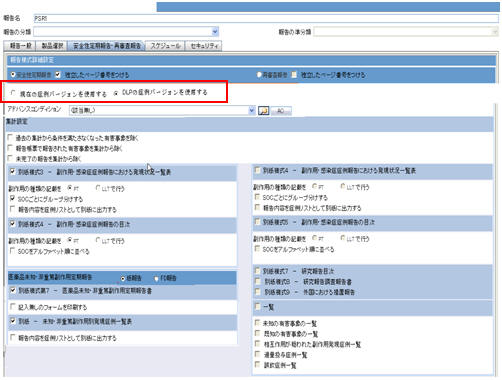
-
These radio button options are displayed only when DLP is enabled.
-
The DLP Configuration radio buttons, Use Current Case Version and Use DLP Case Version are moved to the Report level in the Configuration Print Report of PSR/ReSD, CTPR, and Seiyakukyo reports. The PSR/ReSD Report Configuration print is shown in the screen below:
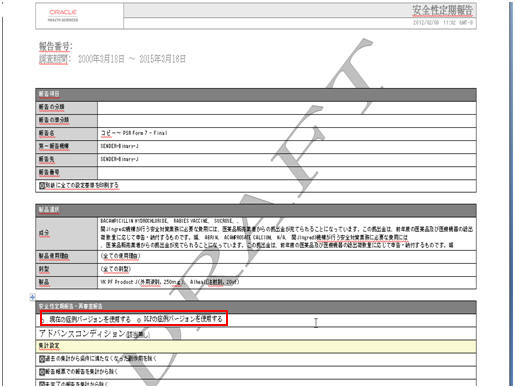
-
The DLP Configuration radio buttons, Use Current Case Version and Use DLP Case Version) are moved to the Report level in the CSPR Report Configuration screen, as shown in the screen below.
-
These radio button options are displayed only when DLP is enabled.
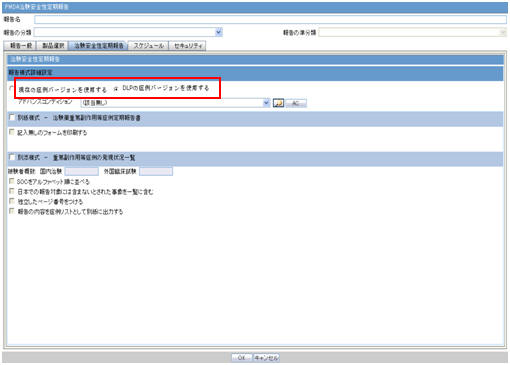
-
The DLP Configuration radio buttons, Use Current Case Version and Use DLP Case Version are moved to the Report level in the CSPR Report Configuration print, as shown in the screen below.
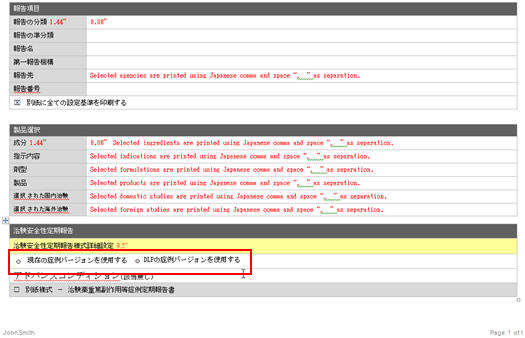
-
The DLP Configuration radio buttons, Use Current Case Version and Use DLP Case Version are moved to the Report level in the Seiyakukyo Report Configuration screen, as shown in the screen below.
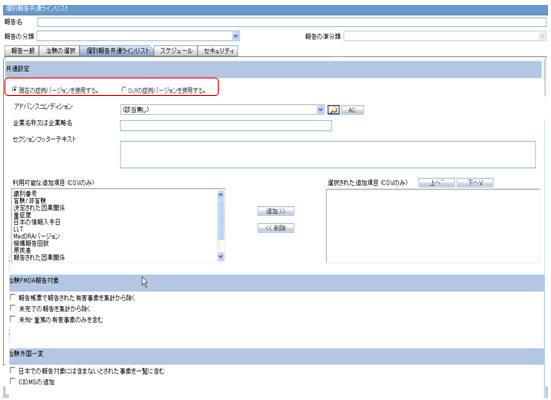
-
These radio button options are displayed only when the DLP is enabled.
-
The DLP Configuration radio buttons, Use Current Case Version and Use DLP Case Version are moved to the Report level in the Seiyakukyo Report Configuration print, as shown in the screen below.
4.2.2 Periodic Reports on DLP
A "global" revision/version in DLP is defined as the qualifying revision/version of case data that is selected as per current logic while running a Periodic report in DLP. Today, this revision/version is based on the Japanese aware dates for Japanese Periodic reports.
A "global cleanup" revision in DLP is defined as the qualifying data clean up revision of case data that is selected as per current logic while running a Periodic report in DLP with option "Next Completed Version".
Note that today this revision/version is based on the Japanese aware dates for Japanese Periodic reports.
When Japanese Periodic Reports (PSR/ReSD, CSPSR, and Seiyakukyo) are generated on DLP using "Last Completed Version", then reportable revision of the case is the last local lock revision that corresponds to the qualifying global revision before the report end date. If there is no local lock corresponding to the qualifying global revision, then the reportable revision of the case becomes the qualifying global revision.
If there is no globally locked version, then the reportable revision of the case is the last local revision that corresponds to the qualifying global revision before the report end date.
When Japanese Periodic Reports (PSR/ReSD, CSPSR, and Seiyakukyo) are generated on DLP using "Next Completed Version", then the system searches for case revision of the last local lock that corresponds to the qualifying global cleanup revision before report end date. If there is no local lock corresponding to the qualifying global revision, then the reportable revision of the case becomes the qualifying global revision.
If there is no globally locked clean-up version, then the reportable revision of the case is the last local revision that corresponds to the qualifying global clean-up revision or qualifying global version or revision, whichever is available, before the report end date.
4.2.3 Upgrade Considerations
In the upgraded database, the DLP Configuration radio buttons at Report level are set as per settings of the parameter at Form level (prior to upgrade) for the PSR/ReSD, CSPR, and Seiyakukyo reports.
4.2.4 Advanced Conditions in J Periodic Reports
-
The following changes are made to the Japanese Periodic reports when Advanced condition is used for filtering cases along with Use DLP Case version.
-
ACs are applied on DLP case data for all forms of PSR/ReSD, CSPR, and Seiyakukyo reports:
If the PSR/ReSD has the Exclude events which don't meet the condition from the past data option checked, the ACs are applied as of the end date of the latest reporting period for the previous reporting period cases in the PSR: Forms 3-4 and ReSD: Forms 4-5.
If the PSR/ReSD has the Exclude events which don't meet the condition from the past data option unchecked, the system fetches the past data as per the existing functionality (without applying AC).
-
-
On clicking the Print button in the PSR Configuration screen for generating Draft or Final PSR report:
-
The system displays the following message:
Report submitted to Background Report Processing. Please view the status of the Report from Reports > Periodic Reports > Background Periodic Report Status screen.
-
Upon generation of the Report, the following links are displayed in the PSR Configuration screen:
FD Reports
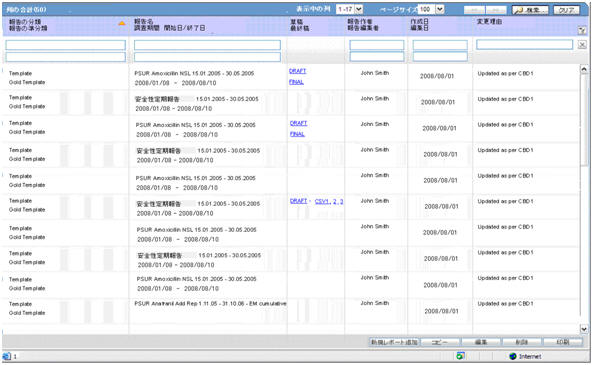
DRAFT link for PSR Paper report(along with Form 7-1) and CSV 1, 2, 3 links for Forms 7-1 and 7-2 OR
FINAL link for PSR Paper report(along with Form 7-1) and CSV 1, 2, 3 link for Forms 7-1 and 7-2
Paper Form report (Existing functionality)
DRAFT link for PSR Paper report OR
FINAL link for PSR Paper report
-
PSR Paper report is opened in IE window on clicking on DRAFT or FINAL links.
-
On clicking the links CSV1, 2, 3, the system displays the corresponding CSV file in MS Excel.
The following table illustrates the PSR ReSD Report Library:
Field separation Comma(,) Text separation Double Quotation("") File Naming convention File name of Final CSV is as follows:COMPANY_ABBREVIATION_NAME-YYYYMMDD-001_1_{Randnum}.csvCOMPANY_ABBREVIATION_NAME-YYYYMMDD-001_2_{Randnum}.csvCOMPANY_ABBREVIATION_NAME-YYYYMMDD-001_3_{Randnum}.csvThe 3 digit number is incremented when more than one report is generated on the same day for the same company. COMPANY_ABBREVIATION_NAME is Company Identifier in Codelist > Reporting Destination > EDI COMPANY_ABBREVIATION_NAME-YYYYMMDD-00X is printed on the bottom of the form 7-1 Paper report.
Randnum is a system generated number and is not printed on the 7-1 Paper report.
The file name for the Draft report is as per Argus Safety.
The following figure depicts the Form 7-1 in CSV format:
-
-
On generating Form 7-1, 7-2 in FD format, 3 CSV files are generated.
-
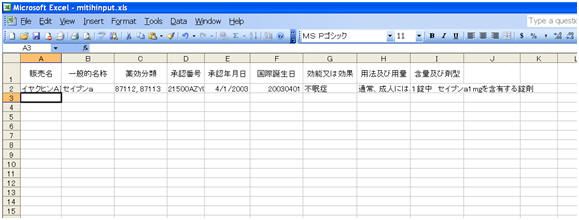
Data printed in CSV file1 is mentioned below:
-
These field values are printed as it is, as in the PSR Form 7-1 PDF Output unless specified.
-
In case of multiple licenses for a product, each product license and all the corresponding field values are printed as a separate row.
-
During Report generation, data is truncated if it exceeds the field length specified in the Report Mapping table below:
English Title Field Length Data Property Trade Name 100J The Trade Name of each product is printed in a separate row and numbering is not used. Generic Name 1000J The Generic Name of each product is printed in a separate row, and numbering is not used. Drug Classification 35AN The Drug Action Classification is printed in a separate row and numbering is not used. License Number 16AN The License Number is printed in a separate row and numbering is not used. Award Date 200J The Japan Award date is entered in the MM/DD/YYYY format.If the Month or Day value is less than 10, it prints without 0. For example, 8/1/2011.Award date of each Product is printed in a separate row and numbering is not used. IBD 8N The International Birth Date is printed in YYYYMMDD format.IBD of each Product is printed in a separate row and numbering is not used. Indication 5000J The logic of printing data is the same as Paper Report- Column - Indication The Indication of Product is printed in a separate row and numbering is not used.
Use 5000J It is populated by default value. Quantity and formulation 5000J The logic of printing data is the same as Paper Report- Column Amount and Formulation
-
-
Data printed in CSV file 2 is as follows:
-
These field values are printed as it is in the PSR Form 7-1 PDF Output, unless specified.
-
During Report generation, the data is truncated if the data exceeds the field length specified in the Report Mapping table below:
English Title Field Length Data Property Investigational time frame 17N The Investigational Unit Timeframe is printed in the following format: YYYYMMDD-YYYYMMDDFor single digit values for MM and DD, leading 0 is added. Aware date 8N Reporting Timeframe starting date (Assigned Date) is printed in the following format: YYYYMMDDFor single digit values for MM and DD, leading 0 is added.
Action taken for ensuring safety and planned action for safety based on the static data 10000J This field is used to enter the description in the output format. Note 5000J This field is used to enter the description in the output format MedDRA version number 4N The latest MedDRA J version configured in Console is printed.For example, 13.1 Report date 8N It is printed as the current database date converted into Japan time zone (by using the common profile switch under Argus J > Reports > Offset from GMT used to calculate Japanese date/time fields (in hours) ) on which the report is being executed.It is printed in the following format: YYYYMMDDFor single digit values for MM and DD, leading 0 is added.The above logic is used in Paper Report also. Address 200J Sender Address: A.3.1.4a-c in the following format: [A.3.1.4c][A.3.1.4b][A.3.1.4a].It is printed as a single line with no line breaks or spaces between these field values. Name 100J Name of person responsible for sending report A.3.1.3b-e in the following format:[Company Name (J)] [A.3.1.3b]space[A.3.1.3e]space[A.3.1.3d]space[A.3.1.3c].A.3.1.3 is printed as a single line with no line breaks.Line break between [CompanyName(J)] and {A.3.1.3}
-
-
Data printed in CSV file 3 is as follows:
-
These field values are printed as it is in the PSR Form 7-2 PDF Output, unless specified.
-
During Report generation, the data is truncated if it exceeds the field length specified in the Report Mapping table below:
English Title Field Length Data Property Number 20AN The logic of printing data is the same as Paper Report- Column Number Preferred Term 100J The logic of printing data is the same as Paper Report- Column Preferred Term MedDRA code 8N The logic of printing data is the same as Paper Report- Column MedDRA Code Gender 1N It is printed based on the Case Form > Patient tab > Gender field value The following is printed:1 for Male2 for Female 3 for Unknown and NULL Age (Value) 5N Age (Value) for B.1.2.2a E2B element Leave the field blank when this is unknown.
Age (Unit) 3N Age (Unit) for B.1.2.2b E2B elementLeave the field blank when this is unknown. AE Occurring Date (Value) 8N The Year and Month of AE is printed in the following format:YYYYMMDDYYYYMMYYYYLeave the field blank when this is unknown. AE Occurring Date (Unit) 3N The Year and Month of AE is printed in the following format:102 for CCYYMMDD610 for CCYYMM602 for CCYYLeave the field blank when this is unknown. Outcome 1N Event outcome based on the E2B code for B.2.i.8 element. Report Type 1N Note, Trade Name 100J This field is used to enter the J drug code. When the code is not available, enter the Product Name (J). Note / Remark 1000J If this non-serious, unlisted adverse event was reported in individual expedited report during the investigational time frame, enter the reports (PMDA Acknowledgement Number). The PMDA Acknowledgement Number is printed as "X-YYYYYYYY" where:
X is the reporting category alphabetic value (J.4a), and
YYYYYYYY is the unique identifier assigned by PMDA for the customer report submission for the corresponding E2b report (J.4b).The PMDA Acknowledgement Number is updated in the following reports/forms":
PSR Form 4 and ReSD Form 5 -> ID Number column
PSR Form 7-2 -> Remark column
Seiyakukyo Line Listing report -> Ack Number column
When the event was reported in multiple safety reports, all the PMDA Acknowledgement Numbers are printed in this section, by printing each ID one below the other in different lines.
Leave the field blank if there is no data to output.Example: 2-05030000 2-05030011

-
-
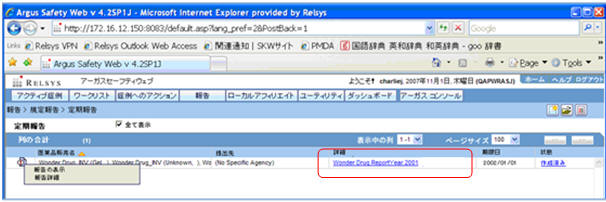
Upon generation of the Final PSR Report in FD format, the Report link is displayed in the Reports > Compliance > Periodic screen.
-
The View Report option for the Japanese Periodic reports is renamed as Edit and View Report.
-
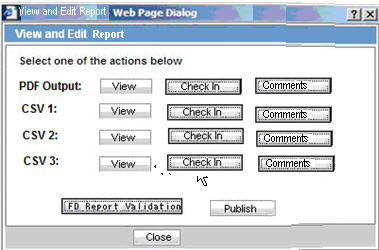
On clicking View and Edit Report for the mixed PDF and CSV PSR Report, the View and Edit dialog box is displayed with separate options to view PDF and CSV report.
-
On clicking View and Edit Report for the PSR Report (Paper format), CSPR, and Seiyakukyo reports, the View and Edit dialog box is displayed by hiding the following:
-
Label: Select one of the actions below
-
Labels: CSV1, CSV2, CSV, and its corresponding Checkin/Checkout and Comments buttons.
-
Button: FD Report Validation
-
View and edit PSR/ReSD
Title
Select one of the actions below:PDF OutputCSV1CSV2CSV3
Fixed labels
View
-
This button is always enabled (regardless of the Checkin/Checkout status) for all users.
-
On clicking the View button for CSV report before/after Publishing, the system opens the CSV Report in Excel in Read-only mode.
-
On clicking the View button for Paper report before Publishing, the system opens the report in Word.
-
On clicking the View button for Paper report after Publishing, the system opens the Final report in PDF format.
Checkin
-
This button allows you to check-in the individual reports.
-
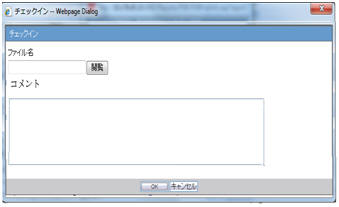
When the Checkin button is clicked for a CSV report, the Argus Safety standard Checkin dialog box is displayed.
-
The Check In dialog box captures the comments for 255 characters.
-
The system allows you to specify only .csv files in the Filename field. If you upload some other file type and click OK, the following standard message application is displayed by this common file upload control:
This is not valid file to upload. Allowed file types are {X} -
When the Check-in button is clicked for a Paper report, the Argus Safety standard Checkin dialog box is displayed to specify the report file name and Checkin Comments.
-
The system allows only .doc files to be specified in the Filename field. If you upload some other file type and click OK, the following standard message is displayed by this common file upload control:
This is not valid file to upload. Allowed file types are {X}
Checkout
-
After the report is checked-in, the Checkin button is changed to Checkout.
-
The Checkout button is disabled after the report is published.
-
Only one user is able to checkout a report.
-
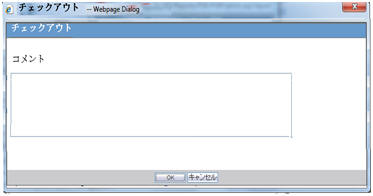
When the Checkout button is clicked for a paper report or CSV report, the system displays the Checkout dialog box:
-
The Checkout dialog box allows you to specify the checkout comments of a maximum size of 255 characters.
-
OK and Cancel buttons are provided in the Checkout dialog box:
-
The Cancel button cancels the Check Out operation and closes the Checkout dialog box.
-
On clicking the OK button for a Paper report, the system opens the report in Microsoft Word in the Update mode.
-
On clicking the OK button for CSV report, the system opens the report in Microsoft Excel in the Update mode.
-
-
After the report is checked out, the Checkout button is changed to Checkin.
-
The same user uses this button to checkin the revised checkout file. For other users, this button becomes disabled as long as it is checked-out by the original user.
-
If you open the Checkout dialog box and completes the Check Out operation after sometime (after another user has already checked - out the report), the following message is displayed:
Report cannot be checked-out as it is in use by another user
FD Report Validation
-
This button is enabled only after all the CSV and WORD reports are checked-in at least once. When this button is clicked, data in all the CSV files are validated.
When there are no error messages, the Argus Safety standard message box with message -
No problem was found in the report is displayed with an OK button.
-
The following validation is executed and the errors are displayed in the form of PDF report.
-
The character check is executed. If there is invalid character specified:
(Item [X-X], Row YY: Invalid character is used)
N (Numeric) - Only 0-9, ., E, +, and - that are used for displaying integers or floating point representation are allowed. Full-width characters are not allowed.
AN (Alpha Numeric) - half-width (1byte) alphabet, Numbers and special characters specified in Argus J common profile switch - Characters to be allowed to use in AN (Alphanumeric) E2B items are used. Full-width (2byte) characters are not used.
J (Japanese) (100J mean 50 double byte J chars) - Full-width characters and AN are used which is same as E2B J validation. Characters specified in Argus J common profile switch - Additional invalid character to be checked in Japanese character validation is considered.
-
Field length check is executed. If there are more characters than the maximum length, the following message is displayed:
(Item [X-X], Row YY: Numbers of characters is exceeding the defined maximum value.)
-
The data format for data items 1-5,1-6,2-1, 2-2, 2-6 in above Data Property is checked, and when format is not correct, the following message is displayed:
(Item [X-X], Row YY: Data format of the column is invalid)
The Validation errors belonging to the data from the CSV files are listed separately. The errors within the CSV file are listed category wise such as Character check, Field length, and Data Property. If there are no errors for all property checks for a CSV file, No problem was found in the report is printed below the CSV#.
-
-
The following validation message in FD Validation report is displayed, when column in CSV does not match with the format specified by Regulation:
The structure of the columns in the CSV file is not in required format
This validation is verified first on clicking FD Report Validation button. If the structure of the CSV file is not in required format, the further validations are not checked for that CSV file.
Publish
-
The Publish button is enabled only after all reports are checked-in at least once.
-
This button is disabled when any of the report is checked out.
-
When the report is not published, you can check in/checkout the report as many times as you want.
-
After the Publish button is executed and the PDF file is created only for Paper report, the system automatically opens only the Paper report on the UI. In addition, the Check-in / Check-out button is disabled and the label is changed to Check-out.
-
The Publish button converts the checked-in Paper report in word document to the PDF file as it is with the same layout. The CSV report that is checked in remains as is and is not converted to PDF.
-
Once Publish is done, the Paper report in word format of the report is no longer available from the Final link and the PDF format overwrites the generated/updated Word format in the database. The Draft link generates the fresh report in Microsoft Word format.
Close
On clicking this button, the system closes the View and Edit Report dialog box.
Comments
-
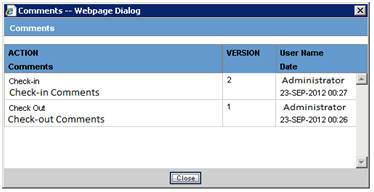
On clicking this button, the Comments dialog box is displayed with the Check-in/Checkout comments.
-
The comments are displayed pertaining to the PDF/CSV for which Comments button is clicked.
-
The Comments dialog box displays the Version Number, Username, Action, Date(Time stamp in User Local date with GMT offset), and Comments entered in the descending order of the Dates.
-
Version of the first check-out is considered version 1.
-
Version number is incremented on every Check-in.
-
Version number is not displayed for subsequent checkout (after first checkout).
-
The Comments dialog box is closed on clicking the OK button.
-
The Report Submission process is the same as other PSR forms.
-
The following Common Profile Switches are moved from Common Profile Switch > ARGUSJ > E2B to Common Profile Switch > ARGUSJ > Reporting in Fresh and Upgraded database.
-
Additional invalid characters to be checked in Japanese character validation.
-
Characters to be allowed to use in AN (Alphanumeric) E2B items.
-
Perform Japanese character validation at E2B Check and E2B Report Generation.
-
-
The Perform Japanese character validation at E2B Check and E2B Report Generation Common Profile Switch is renamed as Perform Japanese character validation at E2B Check, E2B and Periodic Report Generation. This change in the Common Profile Switch is applicable for upgraded database.
Note:
In the future, all periodic report will have the same CSV format options.
4.2.5 Check in/Checkout Enhancements
Checkin/Checkout dialog box changes described above for PSR/ReSD reports are applicable for the following Japanese reports:
-
CSPR
-
Seiyakukyo Report
4.2.6 Other Changes
The TEXT_J data for TRANSLATION_ID ='User Name' is misspelled in LM_TRANSLATION_LABEL table.
4.2.7 Periodic Safety Report
4.2.7.1 Format
The system allows the definition of PSR reports for products. The purpose is to define a fixed set of PSR reports for PRIMARY AGENCY (the various regulatory authorities as optional) and then associate products with these reports. The PSRs can then be scheduled automatically.
PSR applies only for Japanese licenses.
-
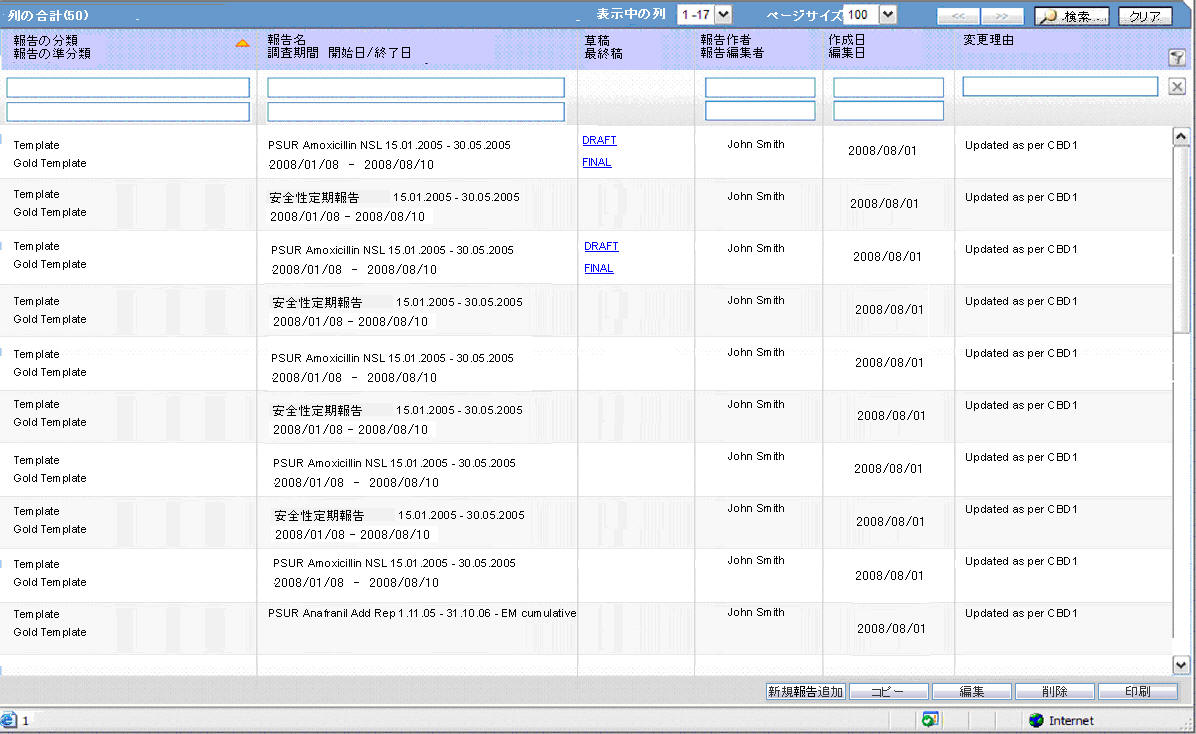
The system contains a configuration screen to define a PSR report. A new menu item, Reports > Periodic Reports > PSR/ReSD Reports is created. When this is clicked, the following page is displayed.
PSR Main Window
-
If the report is being currently opened by another for modification, the system displays the following message:
The report which is selected is being used by XXXX and cannot be modified.
where XXX is the full user name of the user has opened it. -
The labels and buttons related to the CSV files and FD validation reports are hidden in the Edit and View Report dialog box invoked for the PSR Report (Paper format), CSPR, and Seiyakukyo reports.
-
The Create from Template button that is available on Argus E PSUR and CTPR section is not added for Japanese PSR, ReSD configuration.
-
This page displays a list of the PSR reports stored in the system.
-
You can add a new report by clicking on the New Report button.
-
The Copy button makes a copy of an existing report:
When you copy an existing PSR Report, all the configuration of the report including timeframe rows are copied.
Report Name of new copy has copy of in front of the name.
When the configuration is copied, the past dates in the schedule frequency, JAD, IBD, and Assigned date are also copied as read-only. The other sections of the copied configuration are editable.
When you copy an existing non-submitted PSR report, all the configuration including timeframe rows are copied. As the original report is non-submitted, all the timeframe rows are retained as non-editable or editable as they were in the original PSR. The JAD, IBD, and Assigned Date become editable or non-editable depending upon if there are timeframe rows in the copied PSR which are non-editable.
-
The Modify button displays the report definition.
-
The Delete button allows you to delete a report after displaying the following confirmation message:
Proceeding to Yes will remove the report configuration. Proceed? -
Clicking Print, results in displaying a new dialog prior to report preview allowing you to select from several preview or direct export options: Word output.
-
-
The Delete option is only available if there are no final reports generated for the Periodic Reports. The Delete option only hides the report from the list.
-
In the recently executed Periodic Report section, a link is available for the last executed report only if the Report is still available on the Report Server.
-
Clicking New or Modify in the above screen displays the PSR Configuration Window.
-
Once the state of the report is changed to Submitted, you cannot update the configuration of that PSR. This is done by disabling the OK button. For example, when the state is un-submitted, the report is editable again. In addition, when the non-editable configuration is opened, the following warning message is displayed:
This configuration is not modifiable because the status of this Periodic Safety Report is Submitted
When you click OK, this displays the Configuration window.
When you click Cancel, the configuration window is not displayed. -
When the status of the report is Submitted, only the configuration page is printed.
-
The Copy can be done any time while the configuration exists.
When you create PSR after 2nd time, the new copy of the configuration is required. You can then modify the content to print the current PSR.
-
Before saving the PSR configuration, Report Name, Primary Agency, Product Selection, and at least one time frame need to have a value entered.
When these are not entered, the following messages are displayed:
When Report Name is not entered:Report Name is not entered
When Primary Agency is not entered:
Primary Agency is not entered
When selected product is not available:
Product is not selected
When At least one time frame is not entered:
Investigation time frame is not configured
The following message is displayed along with the above error:
It is necessary to enter above information in order to save the configuration
-
When Modify, Copy, Delete, or Print button is clicked without a selection of report, a pop-up is displayed to select a report.
4.2.7.2 PSR /ReSD Details
4.2.7.2.1 Subject of Report
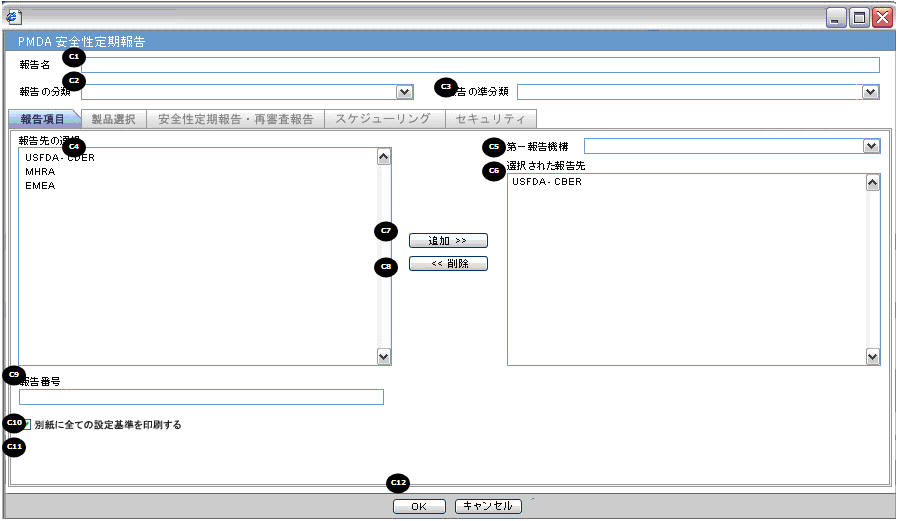
| Field Name | Description |
|---|---|
| Report Name | Manually Input a Name for the Report. It is displayed in the Reports > PSR Reports dialog box. |
| Report Category | Manually Input a Name for a Report Category. It is displayed in the Reports > PSR Reports dialog box. |
| Primary Report Agency | This list contains the Regulatory Authorities configured in List Maintenance > Regulatory Authorities (LM_REGULATORY_CONTACT table).
If there is no Japanese Authority name configured, the English name is listed. The system allows you to select multiple agencies from the List of Agencies such that the report can be submitted to multiple agencies at the same time.The primary agency can be defined in this drop- down.When Japanese Message Profile is not configured in Console J for the selected Primary Agency, or when you add the agency with PMDA E2B R3 (or R2) in the Report Configuration, an error message is displayed as: Selected Primary Agency doesn't have configured Message Profile (I or J) in Argus Console > Reporting Destination/EDI. Once you click OK, the selection of the Primary Agency goes back to the previous selection. This check is executed when you try to go to the other tab, or click OK to save the configuration. |
| Report Number | Manual entry text field that allows you to enter a report number. |
| Print all configuration criteria on separate cover page | Check box to print out the configuration of this report when the report is printed. When this is clicked, the Configuration page is printed at the beginning of the PSR/ReSD word print output. The page numbering of the PSR/ReSD does not include the Configuration page. |
4.2.7.2.2 Product Selection
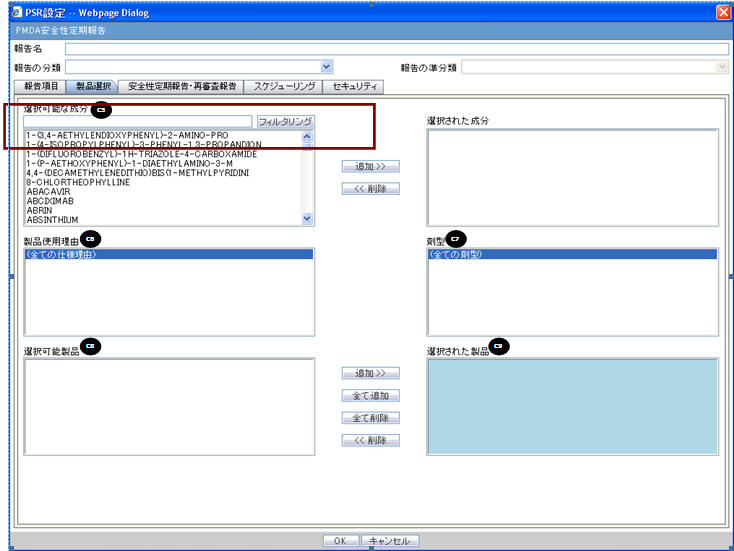
| Field Name | Description |
|---|---|
| Available Ingredients | This list contains the Ingredients used for the products configuration. It is stored in the LM_PF_INGREDIENT_J table once a product has been configured.
The system allows filtering the Ingredients within the available list of Ingredients by a entering the Ingredient name and clicking the Filter button. The search is performed in Japanese ingredient name only. If the name is blank, enter English ingredient name. |
| Filter | |
| Indication | This list contains the Indication configured for the product containing the ingredient in C5 section. Use this to narrow down the products displayed in C8.The system allows to multi-select Indications using the standard Windows functionality of CTRL+CLICK.If Japanese name is not available, the English name is displayed. |
| Available Products | This list is auto-populated once the Ingredient has been selected and displays all products from the LM_PRODUCT J table, containing the ingredient selected in C5 section. The format is Product name (Formulation, Concentration concatenated with the Concentration Units). If Japanese name is not available, the English name is displayed. The withdrawn products are displayed as well. |
| Selected Ingredients | When an ingredient has been selected, all products containing that ingredient are displayed in Available Products section. Multiple Ingredients can be selected at once to form a PSR product family. |
| Formulation | This list contains the formulation configured for the product using the ingredient in C5 section (LM_FORMULATION table). Use this to narrow down the products displayed in C8.The system allows to multi-select formulations using standard Windows functionality of CTRL+CLICK.If Japanese name is not available, the English name is displayed. |
| Selected Products | This list contains products that are selected from the Available Products list (C8). When at least one product is selected, the field has a blue background color. If the license is not withdrawn at the starting date of the PSR investigation timeframe, include the license in PSR and ReSD. |
Additional Information
-
When no Japanese ingredients, indication, or formulation is configured in the Console, the English names are displayed.
-
If the product is added or deleted in the Product Selection section before the PSR/ReSD status is marked as Submitted, it displays a warning pop-up:
If the content of selected product is changed, IBD, JAD, and Assigned date can possibly be changed. Do you want to proceed?). -
If you add or delete the products in the Product Selection section before the first PSR report is marked as Submitted, it updates the IBD, Japan award date, and Assigned Date based on the new product set.
-
When you copy an existing submitted PSR report, and JAD, IBD, Assigned dates are non-editable (you have not clicked the RESET button yet) and If you change the product selection, JAD, IBD and Assigned dates do not change. When this occurs, the following warning message is displayed:
Because the configuration has the record of past investigation period, JAD, IBD, and Assigned Date for scheduling configuration will not be changed by modifying the Selected Products. It is necessary to use the Reset button and re-configure the investigation period if new PSR needs to be created based on modified product selection. -
All the forms printed as PSR and ReSD have only the information regarding the selected product in this configuration window.
4.2.7.2.3 Periodic Safety Report/ReSD
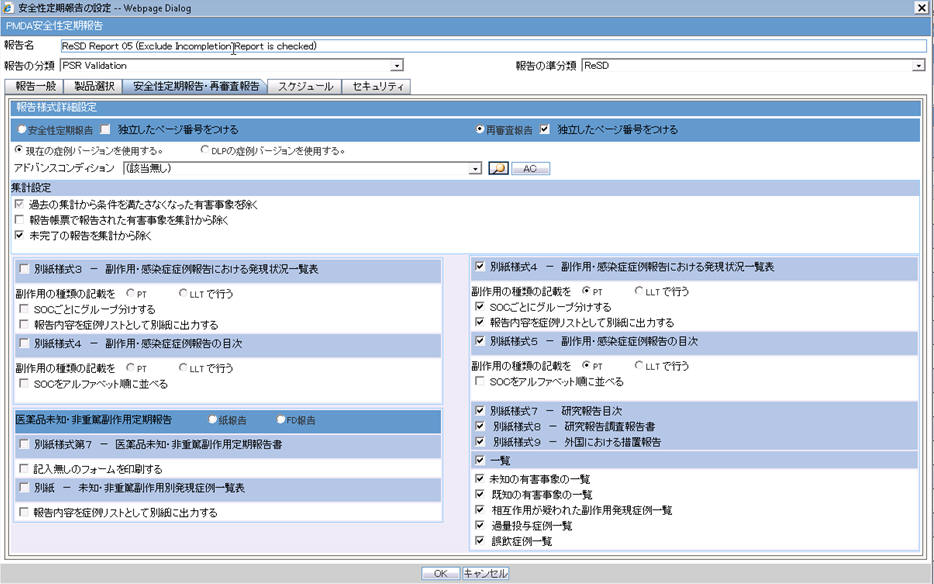
| Field Name | Description |
|---|---|
| Forms configuration | This field represents the main title of the UI. |
| Periodic Safety Report | This radio button is available to select either PSR or ReSD, so that you can create PSR and ReSD independently.This is selected as Default |
| Re-examination Submission Dossier | This radio button is available to select either PSR or ReSD, so that you can create PSR and ReSD independently. |
| Data counting configuration | |
| Count Configuration | |
| Exclude events which don't meet the condition from the past data | When this is checked, the events that were reported in the past PSR, but do not meet the condition at the end of current investigational timeframe are subtractedEvent was downgradedReport was nullifiedWhen this occurs, following message is printed under the first table of form 3:
Among the Events that were count in the past report, there are nullified/became non-reportable during this investigational time frame. This is not checked by default. |
| Exclude events which were reported by Paper report form | When this is checked, the events that were reported by Paper Form during the investigational time frame are excluded.This is not checked by default. |
| Exclude Incompletion report from output | When this is checked, the events reported as incompletion report during the investigational time frame are excluded.This is not checked by default |
| PSR Report Form 3 | |
| Form 3 Selection (Check box) | If this is checked, PSR Form3 is printed.This is checked by default. |
| Print Only the Term Preferred Term (Radio Button)Lower Level (Radio Button) | This field allows you to decide which term (PT or LLT) is to be printed on the form3.PT is selected by default. |
| Classify based on SOC | If this is checked, the PSR Form 3 PT/LLT section is grouped by SOC. In this case, the name of the SOC is listed at top of the section, and --- is used for separator between SOC and PT/LLT. Bold line is used for separating the groups.This is checked by default. |
| Print the report content as Case Listing in the separate paper | When this is checked, the case listing of the report content is printed out on a separate paper. All the configuration reflects the case listing.This is not checked by default. |
| PSR Report FORM 4 | |
| Form 4 Selection (Check box) | If this is checked, the PSR Form4 is printed.This is checked by default. |
| Print Only the Term | This field allows you to decide which term (PT or LLT)is to be printed on the form4.PT is selected by default. |
| Order SOC Alphabetically (Check box) | If this is checked, the SOC is printed in alphabetical order. If not checked, the SOC order is same as that of the MedDRA order.This is not checked as default. |
| Non-Serious Unlisted PSR Report | |
| Drug Non-Serious, Unlisted PSR | This field represents the title of the section. |
| Paper ReportFD Report | Radio buttons:When Paper Report is selected, Paper report format is generated.When FD Report is selected, CSV report format is generated.When FD report is selected, both 7-1 and 7-2 forms are checked in the checkboxes and these cannot be edited. Paper Report is selected by default. |
| PSR Report FORM 7 - 1 | |
| Form 7-1 Selection (Check box) | If this is checked, the PSR Form7-1 is printed.This is checked by default. |
| Print Blank Form | By checking this, the blank form is printed.This is not checked by default. |
| PSR Report FORM 7 - 2 | |
| Form 7-2 Selection (Check box) | If this is checked, the PSR Form7-2 is printed.This is checked by default. |
| Print the report content as Case Listing in the separate paper | When this is checked, the Case listing of the report content is printed out on a separate paper.This is not checked by default. |
| Separate Page Numbering (Check box) | When this is checked, the Page number is independent (starts from 1) for each form.This is checked by default. |
| Use Current Case VersionUse DLP Case Version | This radio button control is only displayed if DLP is enabled.
Use Current Case Version is selected by default.Based on the PSR Configuration for DLP, the report is executed accordingly using latest Case Revision or using the DLP Case Revision. |
| ReSD Report FORM 4 | |
| Form 4 Selection (Check box) | If this is checked, the ReSD Form4 is printed.This is checked by default. |
| Print Only the Term Preferred Term (Radio Button)Lower Level (Radio Button) | Allows you to decide which term (PT or LLT) you want to print on form4.PT is selected by default. |
| Classify based on SOC | If this is checked, the PSR Form 3 PT/LLT section is grouped by SOC. In this case, the name of the SOC is listed at the top of the section, and --- is used as separator between SOC and PT/LLT. Bold line is used for separating the groups.This is checked by default. |
| Print the report content as Case Listing in the separate paper | When this is checked, Case Listing of the report content is printed out on a separate paper.This is NOT checked by default. |
| ReSD Report FORM 5 | |
| Form 5 Selection (Check box) | If this is checked, the ReSD Form5 is printed.This is checked by default. |
| Print Only the Term | Allows you to decide which term (PT or LLT) is to be printed on form4.PT is selected by default. |
| Order SOC Alphabetically (Check box) | If this is checked, the SOC is printed in alphabetical order.
If this is not checked, the SOC order is same as that of the MedDRA order.This is not checked by default. |
| ReSD Report FORM 7 | |
| Form 7 Selection (Check box) | If this is checked, the ReSD Form 7 is printed.This is checked as default. |
| ReSD Report FORM 8 | |
| Form 8 Selection (Check box) | If this is checked, the ReSD Form 8 is printed.This is checked by default. |
| ReSD Report FORM 9 | |
| Form 9 Selection (Check box) | If this is checked, the ReSD Form 9 is printed.This is checked by default. |
| ReSD Report Tabulations | |
| Tabulations | If this is checked, all the tabulations in the section are checked and included in the print.This is checked by default. |
| Tabulation for Unlisted Events | When checked, this tabulation is included in the print.This is checked by default. |
| Tabulation for Listed Events | When checked, this tabulation is included in the print.This is checked by default. |
| Case Overview Tabulations | When checked, this tabulation is included in the print.This is checked by default. |
| Overdose Tabulation | When checked, this tabulation is included in the print.This is checked by default. |
| Accident Exposure Tabulation | When checked, this tabulation is included in the print.This is checked by default. |
Additional Information
-
Separate page numbering always follow the previous form of PSR and ReSD. The following is the order of the page numbering sequence:
PSR Form 3, 4, 7, 7-2, ReSD Form 4, 5, and 789
-
When you select PSR, ReSD using the radio button, the system selects all the forms available for each set of report automatically (PSR - above table 1, 7, 10, and 13) (ReSD - above table 15, 20, 23, 25, 27, and 29). By default, PSR is selected and PSR forms are selected.
-
If the radio button is used to select the PSR form, the previously checked forms on the ReSD are unchecked. If the radio button is used to select the ReSD form, the previously checked forms on PSR are unchecked. While the PSR is selected, the ReSD section is disabled. While ReSD is selected, PSR section is disabled.
-
If you close the configuration and opens again, the configuration on the UI remains as it was at the time of the last operation.
-
All the forms format are based on the Novartis PSR form sample.
-
You are not allowed to change the configuration parameters for PSR Form 3, 4 and ReSD Form 4, 5; if there are any past (non-editable) timeframe rows. If there are past timeframe rows in the scheduling frequency table, configuration of the PSR Form 3, 4 and ReSD form 4, 5 is non-editable.
-
When you check the Print the report content as Case Listing in the separate paper, the Case Line Listing along with the report content is printed on a separate paper.
-
The Case Listing is printed only for the current timeframe for PSR form 3, ReSD Form 4 and PSR Form 7-2.
-
This line listing is independently printed (using separate page) regardless of the Configuration Printing check box status. If you checked the option to print out the configuration content and Case Listing, the Case Listing is appended after the configuration content.
-
The order of the print is Configuration > Form 3 Line Listing > Form 7-2 Line Listing > All the actual reports
-
The Case Line Listing report does not follow the Separate Page option. The Case Line listing is always separated and starting from page 1 for each form.
-
PSR Form 3 Case Line Listing
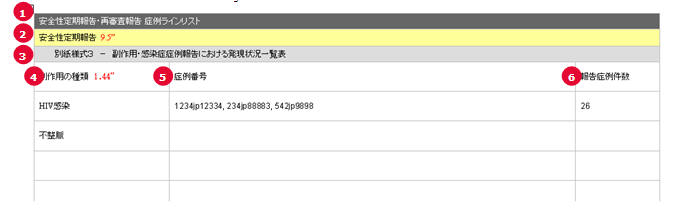
| # |
Field Name | Description |
|---|---|---|
| 1 | PSR/ReSD Case Line Listing | This field represents the section title. |
| 2 | Periodic Safety Report | This field represents the 2nd title. |
| 3 | Periodic Safety Report form #3 | This field represents the title for Form 3 section. |
| 4 | Types of adverse event | The List of AEs are printed using exactly the same rule and order as that of the PSR Form 3. |
| 5 | Case Number | All the reported AE's case numbers are printed in this field. These case numbers are separated by comma. |
| 6 | Number of AEs reported during this timeframe | Number of the AEs available also on the PSR form 3 for this report's investigational timeframe. |
PSR Form 7 Case Line Listing
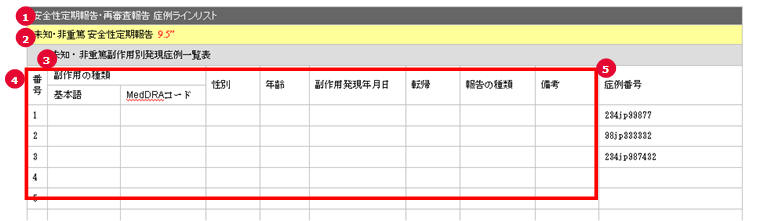
| # |
Field Name | Description |
|---|---|---|
| 1 | PSR/ReSD Case Line Listing | This field represents the section title. |
| 2 | Non Serious, Unlisted Periodic Safety Report | This field represents the 2nd title. |
| 3 | List of non-serious, Unlisted adverse event | This field represents the title for Form 7-2 section. |
| 4 | (form 7 body) | This section is always same as the PSR form 7. |
| 5 | Case Number | The Argus case numbers are added using this field. |
ReSD Form 4 Case Line Listing
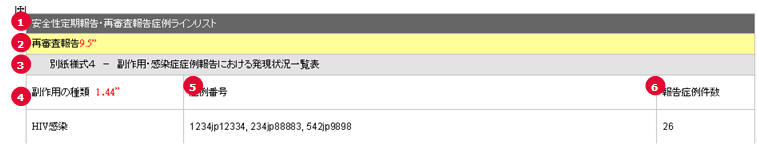
| # |
Field Name | Description |
|---|---|---|
| 1 | PSR/ReSD Case Line Listing | This field represents the section title. |
| 2 | Re-examination submission dossier | This field represents the 2nd title. |
| 3 | ReSD Form #4 | This field represents the title for Form 4 section. |
| 4 | Types of adverse event | List of AEs are printed using exactly the same rule and order as that of PSR Form 3 |
| 5 | Case Number | All the reported AE's case numbers are printed in this field. These case numbers are separated by comma. |
| 6 | Number of AEs reported during this timeframe | Number of the AEs available also on the ReSD form 4 for this report's investigational timeframe. |
4.2.7.2.4 Scheduling
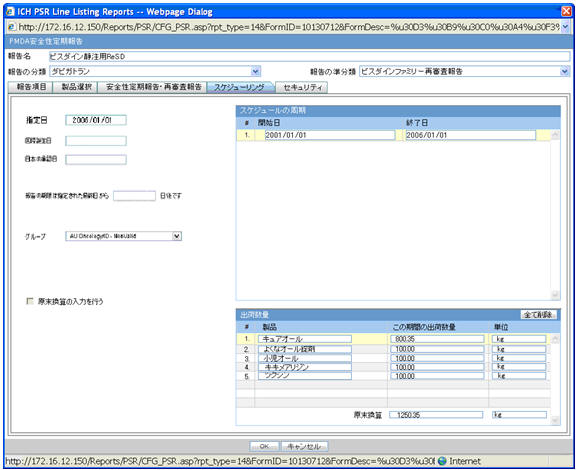
| Field Name | Description |
|---|---|
| Delivery quantity | This field represents the title of the section. This section displays the delivery quantity information for each timeframe row, when that row is clicked. |
| Clear All | When you click this button, all the entered information on this UI is cleared (The data entered by you only. For example, the Delivery Quantity and Delivery Unit for all the products in the Delivery Quantity section is removed but the product names remain in each row.When this is clicked, a confirmation window with the following message is displayed:
If you continue, the entered information is erased. Do you want to continue?. The content in the UI is deleted when you enter OK.No actions are taken on the table if you select Cancel in the message window. |
| Products | Product names used in this PSR appear as default. The names can be changed only at first report generation. From second report, names are read-only unless they are newly added.The maximum text length is 70. |
| Delivery quantity during this period | This field represents the text entry for numbers.The maximum text length is 12 (including the decimal point and number). Only numeric character and decimal point are allowed. |
| Unit | Type ahead functionality with console J Unit (J). For the units data that does not have unit(J), if unit (J) is not available, the field is left blank.You can change the unit only at first report generation. |
| Total Amount | Text entry for number. Only when the entered units are the same, the calculated total from above is displayed in this field. Maximum text length is 15 (including the decimal point and number). Only numeric character and decimal point are allowed. |
| Entering the Delivery Quantity | Delivery quantity table is entered into the report only when this is checked. This is unchecked by default. If this is not checked, the Delivery section of the report is blank (unchecking does not delete the values in UI). |
4.2.7.2.6 Printing PSR / ReSD
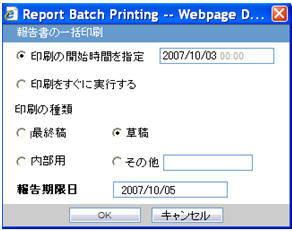
| Field Name | Description |
|---|---|
| Report Batch Printing | |
| Run at | You can specify the time of Reporting using this field. The date entry is in Japanese. |
| Run Now | This field represents the Print now option. |
| Print As | You can select printing option from Final, Draft, Internal, or Other. |
| Other | |
| Due Date | Automatically populate the calculated value by using the due days. |
Generating the Document
Generation of the document is executed using the following process:
-
Click View Report in the Reports > Compliance > Periodic Reports.
-
Check-out the report from the View and edit PSR/ReSD pop-up.
-
Edit the checked-out word document
-
Check-in using the same View and edit PSR/ReSD pop-up.
-
Publish the PDF file (converting from the checked-in word doc)
Printing Requirements
-
The system allows you to Print the Report and update the Report output in the Word document.
-
The change of the configuration for the same investigation timeframe can be done multiple times only before the status is changed to Submitted. The changes in the configuration after the Submitted status of the PSR is applied on the next investigational time period.
-
From the Reports > Compliance > Periodic Reports, selecting View Report allows you to View the PSR/ReSD Report in Word document to upload the report.
-
When the J user opens Reports > Compliance > Periodic Reports from the menu, the Japanese UI is displayed for Periodic report list. The pop-ups for the PSR View and Edit are Japanese.
Field Name Description Trade Name The J UI displays available Japanese data. When Japanese data is not available, the English data is displayed.The Trade Name section displays the multiple trade names using comma separated list of the selected products.The Trade Name displays the original product selected: English or Japanese. If PSUR was created for the English product names, the English product name is used in the list. If the PSR is created with the Japanese product names, the Japanese product name is used in the list.If the selected product in the PSR does not have the Japanese Trade name, the English trade name is displayed for that PSR. Destination The Japanese UI displays the available Japanese data. When Japanese data is not available, the English data is displayed.When Destination is not available, display No specific receiver. Description The Japanese UI displays the available Japanese data. When Japanese data is not available, the English data is displayed. Due Date English date format is DD-MMM-YYYY.Japanese date format is YYYY/MM/DD Status Status appears in Japanese in the Japanese UI, and in English in English UI and can have the following possible values: Deleted
Scheduled
Generated
Approved
Disapproved
Submitted
New Data Available
No Longer Required
-
-
The report output is in Word Document. This works in the similar way as ePSUR publishing where you are allowed to edit the Word output outside the system and once generated, can publish the FINAL Word Document.
-
When the report is generated and never checked out, the center button display Check-out to let you revise the document. At this time, Publish button is disabled.
-
Check-out can be executed by one user only. Other users cannot check in the checked-out files.
-
Once the check-out is executed, the Check-out button is changed to the Check-in button. This button is used to check-in the revised checkout file.
-
When the file is checked-in, the center button is changes to the Check-out button.
-
When the file is not checked-out or not published, you can check-in/check-out the file as many times as you want.
-
When the file is not checked-out or not published, the group which has the access rights to this report can execute the check-in, check-out, and publish.
-
The View button is always enabled (regardless of the check in/check out status). It displays the latest updated Word document or the PDF after final Publish.
-
The Publish button is enabled after at least one time of the check-in action. The Publish button is disabled when the document is checked-out.
-
Once the final Word file is created, the Word document is saved in the repository. All the past PSR word output are saved. When you update the Word document and uploads it, the system overwrites the automatically generated document in the database.
-
Once the Publish button is executed and the PDF file is created, the Check-Out button is grayed out and disabled.
-
Once the document is checked-in, the Check-in button is changed to Check-out, and the Publish button is enabled to publish the PDF report from the Word document.
-
The Publish button converts the checked-in Word document to the PDF file.
-
Once Publish is done, the Word document is no longer available. The Check-in / Check-out button is disabled only when the PSR is published and the label is changed to Check-out.
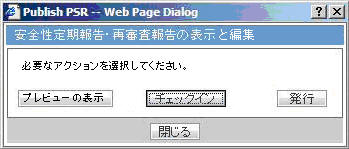
-
-
When the Check-in button is clicked, the following pop-up is displayed to specify the file name. This can be done by either specifying the file name in the text box, or selecting the location using the Browse button.
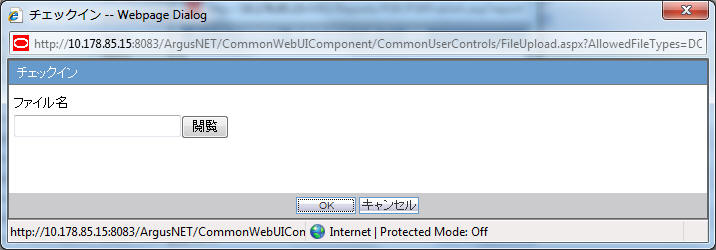
-
If the data is changed manually in the Word document, this change is not applied to the next report. The report needs to be changed again.
-
The configuration of the PSR/ReSD can be edited while the PSR is checked-out. The change is affected only when the PSR is created again.
4.2.8 Clinical Study Periodic Safety Report (now J-DSUR)
Note:
The Clinical Study Periodic Safety Report (CSPSR) is now known as J-DSUR.4.2.8.1 Format
The system allows configure and output J-DSUR defined by the PMDA for products. The purpose is to define a fixed set of J-DSUR for PRIMARY AGENCY (the various regulatory authorities as optional) and then associate products/studies with these reports. The J-DSUR can then be scheduled automatically.The J-DSUR applies only for Japanese licenses.Argus J is capable of output J-DSUR Form 1 and Form 2.
-
The system contains a configuration screen to define a J-DSUR report. A new menu item, Reports > Periodic Reports > J-DSUR ) has been created. When this is clicked, the following page is displayed.
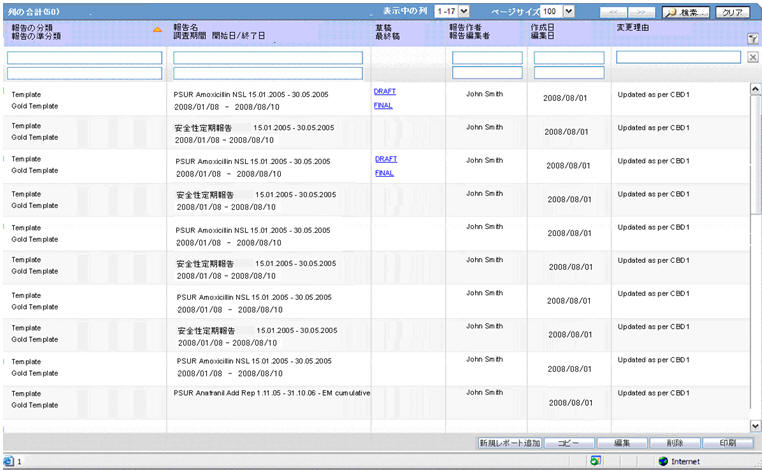
J-DSUR Main Window
-
If the report is being currently opened by another user for modification, the system displays the following message:
The report which is selected is being used by XXXX and cannot be modified.
where XXX is the full user name of the user who has it opened. -
The Create from Template button that is available on Argus E PSUR and CTPR section, is not added for Japanese PSR, J-DSUR configuration.
-
This page displays a list of the CSPSR/J-DSUR reports stored in the system.
-
You can add a new report by clicking the New Report button.
-
The Copy button creates a copy of an existing report.
When you copy an existing CSPSR Report, all the configuration of the report including timeframe rows are also copied, and the CSPSR report gets converted to J-DSUR.
When you copy an existing J-DSUR Report, all the configuration of the report including timeframe rows are also copied.
The Report Name of the new copy has Copy of in front of the name.
When the configuration of already Submitted report is copied, the past dates in the schedule frequency, CSPSD, and Assigned Date are also copied as read-only. Other sections of the copied configuration are editable.
When you copy an existing non-submitted J-DSUR report, all the configuration including timeframe rows is also copied. As the original report was non-submitted, all the timeframe rows are retained as non-editable or editable as they were in the original J-DSUR.
-
The Modify button displays the report definition.
-
The Delete button allows you to delete a report after displaying the following confirmation message:
Proceeding to Yes will remove the report configuration. Proceed?. -
Clicking Print results in displaying a new dialog box prior to Report Preview allowing you to select from several preview or direct export options: Word output.
-
-
The Delete option is only available if there are no Final Reports generated for the Periodic Reports. In any case, the Delete option only hides the report from the list.
-
The generated last executed report is available from a link that fetches the report from the database.
-
Clicking New or Modify displays the J-DSUR Configuration Window.
-
Once the state of the report is changed to Submitted, you are not able to update the configuration of that J-DSUR. This is achieved by disabling the OK button. For example, when the state is un-submitted, the report is editable again. In addition, when the non-editable configuration is opened, the following warning message is displayed:
This configuration is not modifiable because the status of this J-DSUR is Submitted.
When you click OK, the Configuration window is displayed. When you click Cancel, the Configuration window is not displayed. -
When the status of the report is Submitted, only the Configuration page is printed.
-
Copy can be done any time while the configuration exists.
When you create J-DSUR after the second time, the new copy of the configuration is required. You can then modify the content to print the current J-DSUR.
-
Before saving the J-DSUR configuration, Report Name, Primary Agency, Product Selection, and at least one timeframe need to have a value entered.
Else, the following error messages are displayed:
When Report Name is not entered:Report Name is not entered
When Primary Agency is not entered:
Primary Agency is not entered
When selected product is not available:
Product is not selected
When atleast one time frame is not entered:
Investigation time frame is not configured
When Assigned Date is not entered:
Assigned Date is not entered.
Following message is displayed along with the above error:
It is necessary to enter above information in order to save the configuration
-
When the Modify, Copy, Delete, or Print button is clicked without a selection of report, a pop-up is displayed to select a report.
-
If any one Submitted report is present, the Report Configuration is non-editable even if it has multiple generated reports with different states.
-
If you print an existing CSPSR report, it displays only the configuration details.
4.2.8.2 J-DSUR Details
4.2.8.2.1 Subject of Report
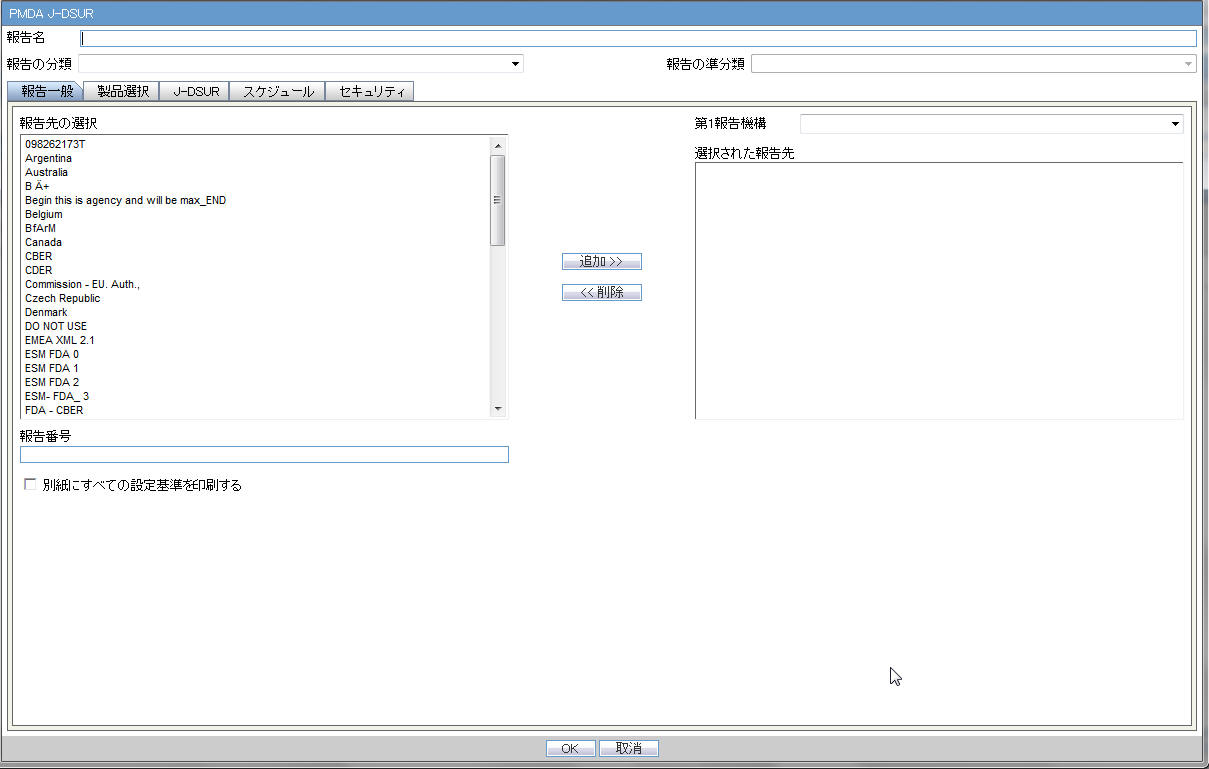
| Field Name | Description |
|---|---|
| Report Name | Manually Input a name for the report that is displayed in the Reports > CSPSR Reports dialog box. |
| Report Category | Manually Input a name for a Report Category that is displayed in the Reports > CSPSR Reports dialog box. |
| Primary Report Agency | This list contains the Regulatory Authorities configured in List Maintenance > Regulatory Authorities (LM_REGULATORY_CONTACT table).
If there is no Japanese Authority name configured, the English name is listed. The system allows you to select multiple agencies from the List of Agencies so that the report can be submitted to multiple agencies at the same time.The primary agency can be defined in this drop-down list.When Japanese Message Profile is not configured in Console J for the selected Primary Agency, the following error message is displayed: Selected Primary Agency doesn't have configured Message Profile (I or J) in Argus Console/Reporting Destination/EDI. Once you click OK, the selection of the Primary Agency goes back to the previous selection. This check is executed when you try to go to the other tab, or click OK to save the configuration. |
| Report Number | Manual entry text field that allows you to enter a report number. |
| Print all configuration criteria on separate cover page | This field represents the check box to print the configuration of this report when the report is printed. When this is clicked, the Configuration page is printed at the beginning of the CSPSR Word print output. The page numbering of the CSPSR does not include the Configuration page. |
If the Japanese Agency Name is not configured in the Console, the English name is displayed on the screen for that agency.
4.2.8.2.2 Product Selection
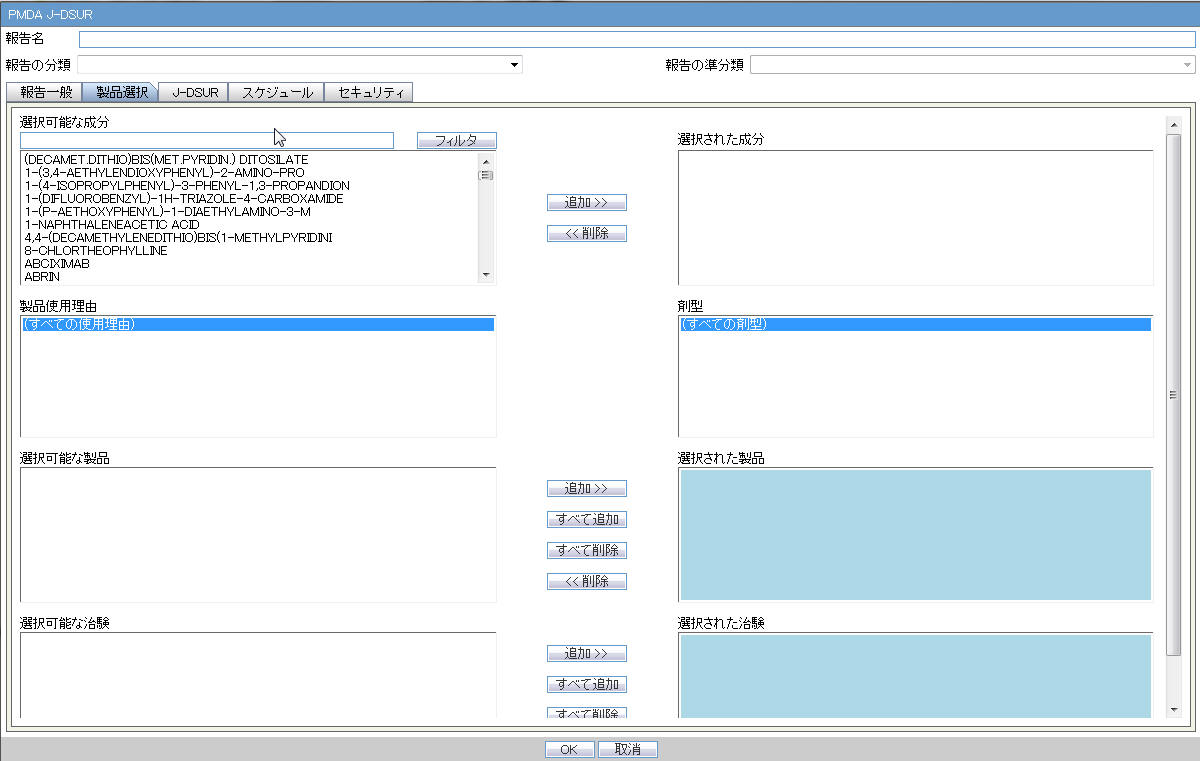
| Field Name | Description |
|---|---|
| Available Ingredients | The system allows filtering the Ingredients within the available list of Ingredients by entering the Ingredient Name and clicking the Filter button.The search is performed in Japanese ingredient name only. If the name is blank, then enter English ingredient name. |
| Indication | This list contains the Indication configured for the product containing the ingredient in C5 section. Use this to narrow down the products displayed in C8.The system allows to multi-select Indications using standard Windows functionality of CTRL+CLICK.If Japanese name is not available, display the English name |
| Available Products | This list is auto-populated once the Ingredient has been selected and displays all products from LM_PRODUCT J table, containing the ingredient selected in C5 section. The format is Product name (Formulation, Concentration concatenated with the Concentration Units). If the Japanese name is not available, the English name is displayed.
The withdrawn products are displayed as well. |
| Selected Ingredients | This list contains the Ingredients used for the Products Configuration. It is stored in the LM_PF_INGREDIENT_J table once a product has been configured. When an ingredient has been selected, all products containing that ingredient are displayed in Available products (C8) section. Multiple Ingredients can be selected at once to form a CSPSR product family. |
| Formulation | This list contains the formulation configured for the product using the ingredient in C5 section (LM_FORMULATION table). Use this to narrow down the products displayed in C8.The system allows to multi-select formulations using standard Windows functionality of CTRL+CLICK.If Japanese name is not available, the English name is displayed. |
| Selected Products | This list contains products that are selected from the Available Products list (C8). When at least one product is selected, the field has a blue background color. If the license is not withdrawn at the starting date of the J-DSUR investigation timeframe, include the license in J-DSUR.
Data Entry Discipline: When the new product is available because the new product with the same ingredient released in the J-DSUR, you need to manually set up the new configuration. When no Japanese ingredients, indication, or formulation is configured in the Console, the English names are displayed.When you copy an existing submitted J-DSUR report, the Assigned dates remain editable. In such a scenario, if you change the product selection, the Assigned dates do not change. All the forms printed as J-DSUR have only the information regarding the selected product in this Configuration window.When a product is moved to Selected Products field, but this does not have the Clinical Compound Number, the following error message is displayed: Selected product doesn't have a Clinical Compound Number. It is necessary that the study product license has at least one Clinical Compound Number in order to include in the report. If License # as well as Clinical Compound Number is not available, then for J users, if Trade Name (J) is available then it will display <License Authorization Country> (<License Type>) <Trade Name (J) from Console J>. Else <License Authorization Country> (<License Type>) <Trade Name from Console> is displayed. |
| Available Studies | Lists the available studies. |
| Selected Studies | Lists the selected studies. |
Available/Selected Studies multi-select shuttle controls
-
Available Studies field dynamically lists all the company configured studies in Console, which consists of the selected J-DSUR products. The Study ID (J) value from Console > Study Configuration must be listed.
-
These 2 shuttle controls have Add, Add All, Remove, and Remove All buttons to Select/Remove studies between Available and Selected Studies fields.
-
If any product is added/removed from the Selected Product field, the Available and Selected Studies fields are also updated dynamically.
-
The Selected Studies field is displayed in blue background, same as that of the Selected Products fields.
-
If Selected Studies fields do not contain any study, clicking on OK button displays the following error message and brings the focus back on the Selected Studies field:
At least one domestic study must be selected in order to proceed with the configuration.
4.2.8.2.3 J-DSUR
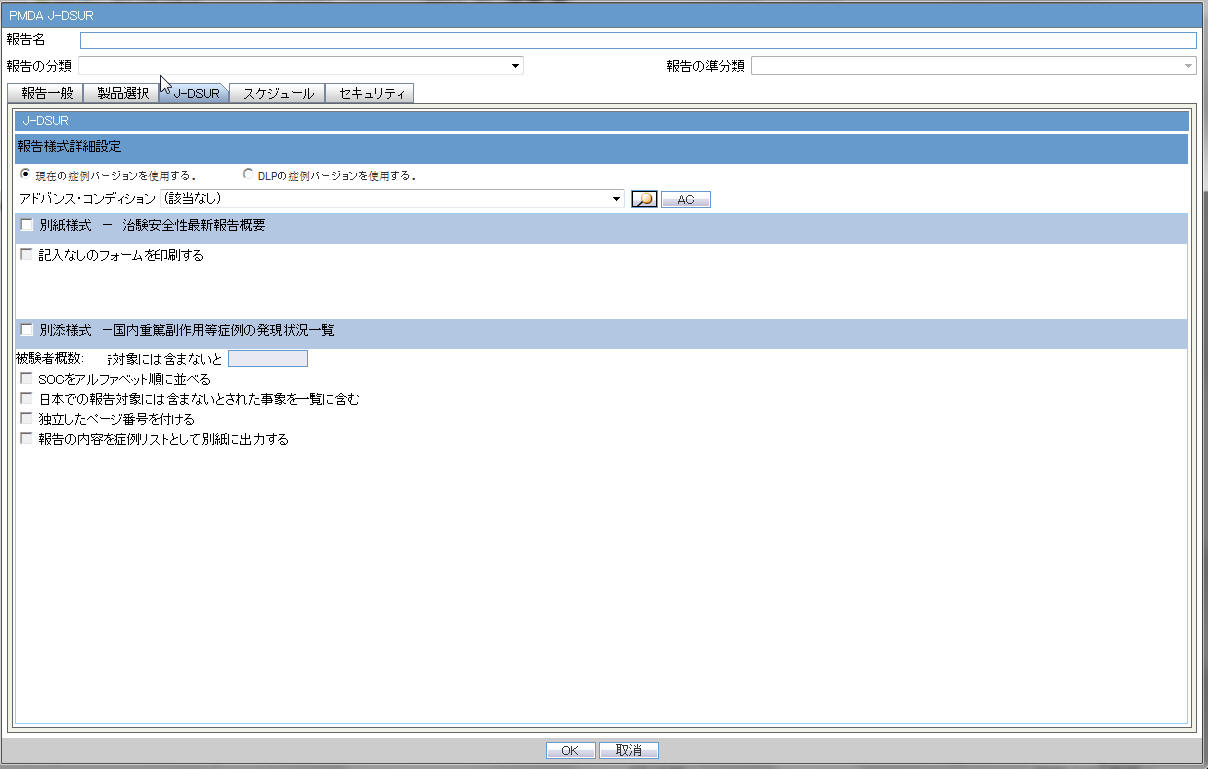
| # |
Field Name | Description |
|---|---|---|
| 1 | Forms configuration | This field represents the main title of the UI. |
| 2 | J-DSUR | This field represents the title of the set of configuration forms. |
| J-DSUR Form 1 | ||
| 3 | Report form - Clinical Trial Safety Update Report Overview | This field represents the title of the form to be configured. |
| 4 | Print Blank Form | This checkbox is used to print the blank form. |
| J-DSUR Form 2 | ||
| 5 | Report form - Serious AE Case Occurrence Status Listing | This field represents the title of the form to be configured. |
| 6 | Number of subject: Study__ | This is a manual data entry (Numeric only) field. You can enter total number of subject for this entire clinical study for domestic.The Maximum digits for this number is 7 for both fields. |
| 7 | Order SOC Alphabetically (Check box) | If this is checked, the SOC is printed in alphabetical order (alphabetically based on their English names). If not checked, the SOC order is same as that of the MedDRA order. |
| 8 | Include foreign AE marked as "Not include for the report in Japan" (Check box) | When this is checked, the system includes AEs in the foreign cases that have flags for Not include for the report in Japan (available in the Case Form > Event tab) |
| 9 | Use Current Case VersionUse DLP Case Version | This radio button control is only displayed if DLP is enabled.
Use Current Case Version is selected by default.Based on the J-DSUR configuration for DLP, the report is executed accordingly using latest case revision or using the DLP case revision. |
| 10 | Separate Page Numbering (Check box)
Note: When separate page numbering is not used, it always follows the previous form of J-DSUR. Order of the page numbering sequence is following: J-DSUR Form Cover, Line Listing |
When this is checked, the page number becomes independent (starts from 1) for J-DSUR Line Listing. |
| 11 | Print the content of the report as case listing on separate page | If this is checked, Case Listing of the J-DSUR Line Listing content is printed on a separate page.
Note: The Case Listing is printed after the configuration content, and using following style guide template for listing. |
| 12 | OK | |
| 13 | Cancel |
StyleGuide_CaseList.doc
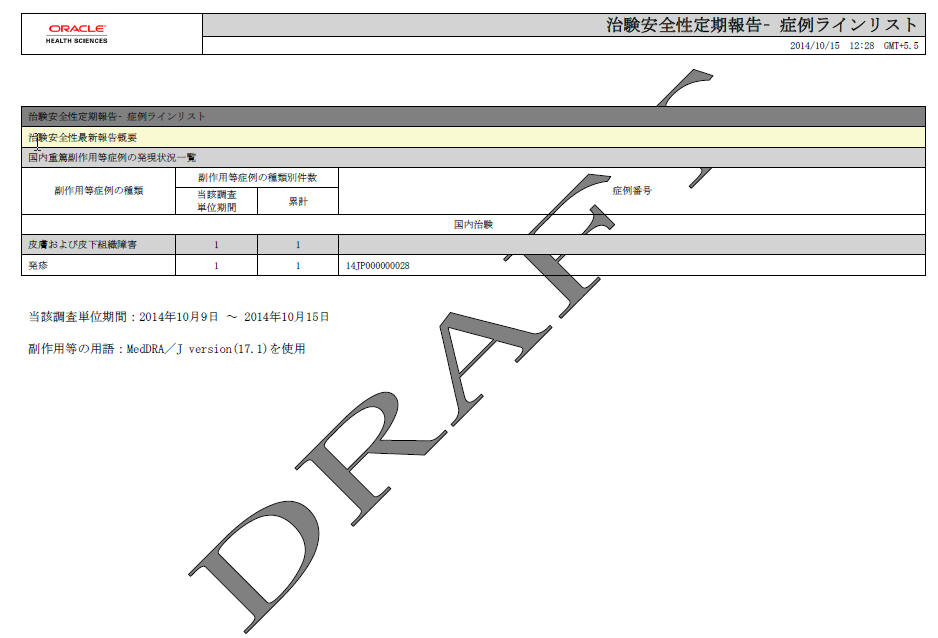
| # |
Field Name | Description |
|---|---|---|
| 1 | Periodic Safety Report - Case Line List | Static text |
| 2 | Clinical Study Serious Event Case Periodic Report | Static text |
| 3 | Serious AE, etc. Case Occurrence Status Line Listing | Static text |
| 4 | AE, etc. Case classification | Static text |
| 5 | AE, etc. Case Count | Static text |
| 6 | This investigation period | This column has the same output as the printed J-DSUR |
| 7 | Cumulative Total | This column has the same output as the printed J-DSUR |
| 8 | Case Number | Case Numbers that have counted events are listed with the comma used as a separator. |
| 9 | Clinical Study | Static text. This section only considers the cases, which contain the studies configured as Selected Studies in the Product Selection tab. |
| 10 | SOC | This field has the same output as the printed J-DSUR.The Case Listing is not printed for this section. |
| 11 | Event sectopm | This field has the same output as the printed J-DSUR.The Case listing is printed for this section. |
| 12 | This investigation time frame: YYYY?MM?DD????YYYY?MM?DD? - TBD | This field has the same output as the printed J-DSUR. |
| 13 | Terms for AE: MedDRA/J version ( ) was used. | This field has the same output as the printed J-DSUR. |
4.2.8.2.4 Scheduling
| Field Name | Description |
|---|---|
| Clinical Study Plan Submit Date | This is a manual data entry text field. It is an editable text field.
If OK is clicked, the new value remains in the field. If Cancel is clicked, the original value is replaced with the entered value in the field.CSPSD is an editable field, if the J-DSUR is not in Submitted state and it does not have any previous non-editable timeframes.Once a J-DSUR is marked as Submitted, this field becomes non-editable. |
| Report is due ___days after specified end date | This option allows you to specify the due date of the J-DSUR, xx days after the end date specified for the scheduling period. The value entered in this field is added to the current end date and displayed in the printing UI as due date. |
| Group | This option allows you to specify which Argus group is responsible for the Periodic report, once scheduled by the application. The report appears on the designated group's worklist for Reports. |
| Frequency of the schedule | |
| Start Date | This field is a date entry field for the reporting timeframe starting period. It allows you to enter the starting date of each J-DSUR timeframe starting period. The first Report Timeframe Start Date is auto-calculated as per the Assigned Date. The Start Date for the first timeframe is editable till the report is submitted.
Start Date of the new row always has the next date of the previous End Date pre-populated as read-only, and End Date can be added only one at a time.The Start Date of the J-DSUR is auto-populated and reset as per the Assigned Date value, only if the first timeframe row (first start as well as end date) is editable. The Start Date of the J-DSUR is not updated based on the Assigned date, if this first timeframe row is non-editable.Whenever Start Date is auto-updated and end date is already specified, the system displays the following warning message: Start Date for the reporting period has changed. Please update the End Date accordingly.When you enter the Start Date which is older than the latest End Date (previous row), the system displays the following error message: Start Date must be same day, or after the latest end date. By clicking OK, the wrong Start Date is removed. Note: The J-DSUR contains the number of events from Submitted to the Japanese Agencies and non-reportable events within the Reporting Period. |
| End Date | This field is a date entry field for the reporting timeframe ending period. It allows you to enter the ending date of each J-DSUR timeframe starting period. This is an editable field.When you enter the End Date which is earlier than the same timeframe's Start Date, the system displays the following error message:
End date must be after the Start Date By clicking OK, the wrong end date is removed. Note: The J-DSUR contains the number of events from Submitted to the Japanese Agencies and non-reportable events within the Reporting Period.The System does not allow you to edit the previous reporting period and only allows to edit the current reporting period. |
| Add | You can add editable row by clicking this button. You can add only 1 reporting timeframe which can remain in Editable state in a J-DSUR in single configuration. All the previous reporting timeframes are always in non-editable state.By default, in a freshly configured J-DSUR (non-submitted, non-copied), there is no timeframe row to begin with until you add one row using the Add button.Whenever the first timeframe row is added, the Start Date is auto-populated based on the Assigned Date.When you generate a J-DSUR, it is generated based on the last editable timeframe row.Once the report is marked with the Submitted status, all the timeframe rows are marked as Non-editable.If you do not have any editable timeframe row available in the J-DSUR, only then Add button is enabled. Else, it is always disabled.The Add button adds editable fields for Start and End date. Each row has a number that starts from 1 and is incremented for each timeframe. |
| Delete | You can delete the editable row by clicking this button. The Delete button cannot delete the non-editable row. For removing previous non-editable rows, you can use the RESET button. This makes sure that you do not delete any timeframe rows which J-DSUR has already submitted. When this is selected, the following warning message is displayed to confirm the operation:
Would you like to delete the highlighted row? |
| Reset | When this button is clicked, all the existing timeframe rows (editable as well as non-editable) are deleted. Else, the past Start and End date records are non-editable. When the Reset button is clicked, the following warning message is displayed:
By using Reset, all the past data will be removed. Do you want to proceed? If you click OK, proceed with the reset. If you select Cancel, leave the past data in the schedule table.When you make copy of a configuration, the Assigned Date, CSPSD, and CTPCSD are non editable unless the Reset button is used. |
4.2.8.2.6 Printing CSPSR
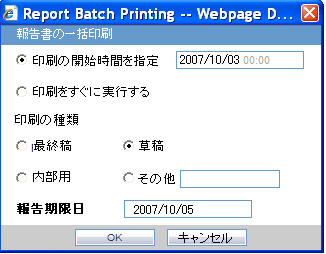
Generating the Document
The generation of the document is executed using the following process:
-
Click View Report in Reports > Compliance > Periodic Reports
-
Check-out the report from the View and edit J-DSUR pop-up.
-
Edit the checked-out Word document
-
Check-in using the same View and edit J-DSUR pop-up
-
Publish the PDF file (converting from the Checked-in Word document)
Printing Requirements
-
The system allows you to print the report and update the report output in the Word document. The watermark is printed on the report when the Draft, Internal or <Other> is selected for printing.
-
The change of the configuration for the same investigation timeframe can be done multiple times only before the status is changed to Submitted. The changes in the configuration after the Submitted status of the J-DSUR is applied on the next investigational time period.
-
From the Reports > Compliance > Periodic Reports, selecting View Report allows you to view the J-DSUR Report in the Word document to upload the report.
-
When J user opens Reports > Compliance > Periodic Reports from the menu, Japanese UI is displayed for Periodic report list. The pop-up for the J-DSUR view and edit is in Japanese.
# Field Name Description 1 PERIODIC REPORTS Lists the Periodic Reports. 2 View All Enables you to view all the reports. 6 Trade Name The Japanese UI displays available Japanese data in this field. When Japanese data is not available, the English data is displayed. 7 Destination The Japanese UI displays available Japanese data. When Japanese data is not available, the English data is displayed.When Destination is not available, No Specific Receiver is displayed. 8 Description The Japanese UI displays available Japanese data in this field. When Japanese data is not available, the English data is displayed. 9 Due Date The English date format is DD-MMM-YYYY.The Japanese date format is YYYY/MM/DD. 10 Status The Status appears in Japanese in Japanese UI, and in English in English UI and can have the following possible values: Scheduled
Generated
Approved
Disapproved
Submitted
New Data Available
No Longer Required
-
Trade Name section:
The Trade Name section displays the multiple trade names using comma separated list of the selected products.
The Trade Name displays the original product selected: either English or Japanese. If PSUR was created for the English product names, the English product name is used in the list. If the J-DSUR is created with Japanese product names, the Japanese product name is used in the list.
If the selected product in the J-DSUR does not have the Japanese Trade Name, the English Trade Name is displayed for that J-DSUR.
-
-
The report output is a Word Document. This works in the similar way to ePSUR publishing where you are allowed to edit the Word Output outside the system and once generated, can publish the FINAL Word Document.
-
When the report is generated and it has been never checked out, the center button displays Check-out to revise the document. At this time, the Publish button is disabled.
-
Check-out can be executed by one user only. Other users cannot check-in the checked-out files.
-
Once the check-out is executed, the Check-out button is changed to the Check-in button. This button is used to check- in the revised checkout file.
-
When the file is checked-in, the center button becomes the Check-out button.
-
When the file is not checked-out or not published, you can check-in/check-out the file as many times you want.
-
When the file is not checked-out or not published, the group which has access rights to this report can execute the Check-in, Check-out, and Publish.
-
The View button is always enabled (regardless of the Check-in/Check-out status). It displays the latest updated Word document or the PDF after final Publish.
-
The Publish button is enabled after the check-in has been performed atleast once. The Publish button is disabled when the document is checked-out.
-
Once the final Word file is created, the Word document is saved in the repository. All the past J-DSUR Word outputs are saved. When you update the Word document and upload it, the system overwrites the automatically generated document in the database.
-
Once the Publish button is executed and the PDF file is created, the Check-Out button is disabled.
-
Once the document has been checked-in, the Check-in button is changed to Check-out, and the Publish button is enabled to publish the PDF report from the Word document.
-
The Publish button converts the checked-in word document to the PDF file.
-
Once Publish is done, the Word document is no longer available. The Check-in/Check-out buttons are disabled only when the J-DSUR is published and the label is changed to Check-out.
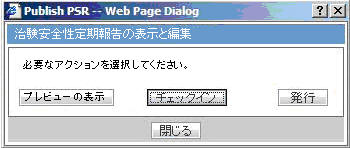
-
-
When the Check-in button is clicked, the following pop-up is displayed to specify the file name. This can be done by either specifying the file name in the text box, or selecting the location using the Browse button.
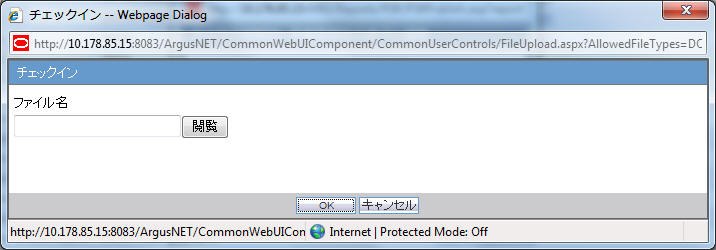
-
If the data is changed manually in the Word document, this change is not applied to the next report. You need to change the report again.
-
The configuration of the J-DSUR can be edited while the J-DSUR is checked-out. The change is affected only when the J-DSUR is created again.
4.2.8.3 J-DSUR - Cover
Summary: The J-DSUR Cover is the cover page of the J-DSUR Line Listing, and output includes the information of the configured product. This form does not contain any particular case data.
| Field Name | Description |
|---|---|
| Clinical Study Drug Serious Adverse Events etc. Case Periodic Safety Report | |
| Form | |
| Clinical Compound Number | This field represents the Clinical Compound Number of the selected product from Console Business Configuration/Product/License.Use ([1],[2],[3]… ) numbering format for multiple Clinical Compound Numbers. |
| Clinical Study Plan Submit Date | This field is a manual data entry field. If this field is changed by the user and this date is later than the "Clinical Trial for Partial Change Plan Submit Date", it displays a message that CSPSD cannot be after CTPCSD. |
| Ingredient Name | The generic name is entered in this field. If the license is withdrawn before the starting date of the CSPSR timeframe, the generic name is not displayed.If Japanese name is not available, this field can be left blank. |
| Dev International Birth Date | This field is auto-populated as per the earliest Console > Product > DIDB field for the J-DSUR products. This date is used to auto-populate the first J-DSUR Start Date on the "Frequency of the schedule" table on the right side of the UI. |
| Trade Name | The Trade Name is based on the selected product in the Configuration window. If there are multiple products (different amount, formulation, etc), use ([1],[2],[3]… ) numbering with the trade names. You can use Trade name (J Drug code). When the code is not available in the code lists, the code is not necessary. If there is a product that has multiple Japanese licenses, all of the trade names must be listed.This field is entered when the selected product is the marketed product (Clinical Trial for Partial Change), but if this is for the Study drug, this field is left blank. |
| International Birth Date | This field represents the IBD from the Code List Product.
Use ([1],[2],[3]… ) numbering for multiple names. |
| Japan Award Date | Code List Licenses -Japan Award date
Use ([1],[2],[3]… ) numbering for multiple names. All the Japanese award dates are listed in this field. The Japanese Award date can be retrieved from License award date field for Japanese license Code List.If the license is withdrawn before the starting date of the CSPSR timeframe, the Japan Award Date is not displayed.This field is entered when the selected product is the marketed product (Clinical Trial for Partial Change), but if this is for the Study drug, this field is left blank. |
| Amount and Formulation | This field represents the amount of the ingredients and formulation (from Dosage info) of the product. If multiple formulations are reported in the same form, enter the information for each formulation. The Strength and unit of the product, and formulation are listed in the following format:"Strength""unit" "Formulation"If the strength data is not available, only the formulation is listed.Use ([1],[2],[3]… ) numbering for multiple information.If Japanese name is not available, the section is left blank. |
| Number of the CSPSR report sent for this ingredient | This field denotes the total number of CSPSR report sent to PMDA including the current time. This starts from 1, 2, 3… |
| Expected Indication | Enter Primary Indication from the Business Configuration/Product/Primary Indication.Use ([1],[2],[3]… ) numbering for multiple Indications.If Japanese name is not available, this section is left blank. |
| Expected Use and Dosage | The information is not printed in this section. You need to enter the information in the Word document output. |
| Investigational Unit Timeframe | This field is used to enter the Time frame Start Date and Time frame End Date for this report. This denotes the Investigation timeframe for this report. |
| Study Phase | Enter Console J > Business Configuration > Studies > study phase J using this field.
When there are multiple clinical studies that use the same Clinical Compound number, use ([1],[2],[3]… ) numbering and list each study phase of all the studies using comma separated string against each number. It only considers the studies configured as Selected Domestic Studies in the Product Selection tab.If Japanese name is not available, this section is left blank. |
| Approval Status in major developed countries | This field represents Approval status in major developed countries excluding Japan.
The value in this field is printed based on the earliest award date of marketed licenses (excluding the hidden link licenses for these products) for the J-DSUR product(s) for configured countries in profile switch Argus J > Reporting>'Major Developed Countries for Approval Status in J-DSUR' (comma separated A2 country codes). Use numbering ([1],[2],[3]… ) for multiple products. The country is printed with its A2 country codes and Aware Date year shall be printed in YYYY format. Example: If in a JDSUR report we have 2 products P1 and P2 with P1 having 2 CCN - CCN1, CCN2 and P2 having 2 CCN - CCN3, CCN4 then approval status are printed as - ([1], [2]) US - YYYY, UK - YYYY, DE - YYYY ([3], [4]) US - YYYY, UK - YYYY, DE - YYYY Where US, UK and DE are configured country codes in the profile switch in this particular order only. Only Marketed Licenses related information are printed here. If Marketed License is not available then do not print any value for that country not even the A2 country code for that country. The values are printed in the same order as A2 codes entered by user in the common profile switch. |
| Status of the Serious AE, etc. case occurrences | This field is used to enter TBD (As described in the Line Listing report). |
| Safety action based on the data analysis and safety countermeasure for the future | This field is left empty to enter the description in the output format. |
| Note | This field is left empty to enter the description in the output format. |
| From above reason, this reports Clinical Serious Case AE Periodic Reports | |
| YYYY?MM?DD? TBD | The Date report is created in the Japanese format. It is printed as the current database date converted into Japan time zone (using the Common Profile Switch under Argus J > Reports > Offset from GMT and is used to calculate Japanese date/time fields (in hours)) on which the report is being executed. |
| Address | This field represents the Address of the sender (A.3.1.4a-c). Format: TBD[A.3.1.4c][A.3.1.4b][A.3.1.4a]
A line break is inserted if the address is reached to the maximum printable length for the line in the print. The address line can be 3 lines in one page, and if the 3rd line reaches the maximum length, it is printed to the next page.No spaces are used between the populated values.The Address and the Name are printed from center of the page (same as PMDA Expedited Reports Name and Address print) |
| Name | This field represents the Sender Identifier(Company Name (J), A.3.1.3b, A.3.1.3c, A.3.1.3d, A.3.1.3e)
Format: TBD[Company Name (J)] [A.3.1.3b]space[A.3.1.3e]space[A.3.1.3d]space[A.3.1.3c]The print start point of [A.3.1.3b] is the same as [Company_Name_(J)] in above line.For the second line, empty space is printed between the populated valuesA line break is inserted, If A.3.1.2 reaches the maximum printable length for the line. There can be 2 lines in one page. And if the 2nd line reaches the maximum length, it is printed to the next page.. A line break is inserted, If combination of [A.3.1.3b] [A.3.1.3e] [A.3.1.3c] reaches the maximum printable length for the line. There can be 2 lines in one page. And if the 2nd line reaches the maximum length, it is printed to the next page. The SENDERMIDDLENAME is not printed if it does not existThe Address and Name is printed from center of the page (same as PMDA Expedited Reports Name and Address print) |
| Receiver identifier | PMDA Receiver identifier A.3.2.2d-f in _____ section.This is printed as follows:Receiver Identifier (A.3.2.2a, A.3.2.2c, A.3.2.2d, A.3.2.2f)
Format: [A.3.2.2a][A.3.2.2c]space[A.3.2.2f]space[A.3.2.2d]space? - TBD |
Additional Information
-
This cover page has Japanese license information only.
-
If all the information is the same through all the numbered items, enter the numbers with one information. When there is no data available, skip the data and only print the serial numbers for which the values are present.
-
If the license is withdrawn before the Start Date of the J-DSUR time frame, all the product information as well as the license information is not displayed.
-
The overflow data is printed on the next page on the copy of this cover page that does not have any data other than the overflow data.
-
For Ingredient Name, Indication, Study Development Phase, Amount, and Formulation, if source console values are not configured, fields on the paper form are left blank.
4.2.8.4 J-DSUR - Form 2
Summary: J-DSUR - Form 2 is the report of events that are serious (both Reported and Not Reported) during the specified investigational time period for Study drugs (including Study for partial change of the Marketed drugs).
-
The past record is included in the report as cumulative Total Number of SOC and Terms.
-
The number of the serious events which are either Reported or Not Reported to the PMDA within one investigational timeframe is the content for the report.
-
Regardless of Incomplete or Complete report, all the events during the investigational timeframe are listed.
-
If there are multiple events in one case, count each Reportable event separately. For example, if there are 2 same PTs available in the same case, the count is 1 SOC and 2 PTs.
-
A case is included in the report for the current timeframe only if a significant Japanese follow-up is received for the case in the same timeframe. If there are no significant updates for the case, this case is not counted for the current period.
-
If there are multiple follow-up updates (Significant or Non-significant) to a qualifying case in the same investigational time frame, count the events based on latest information on the events in the case and exclude the Deleted events during the timeframe. If the event is entered as Serious, but later in the same timeframe, this is changed as Non Serious, this event is not counted.
-
The MedDRA version is the latest MedDRA J version at the time of report creation.
-
The configured products that fall in DRUGCHARACTERIZATION = 1 (SUSPECTED) or 3 (INTERACTION) are collected. 2 (Concomitant) is not included.
-
The date cut off for the data inclusion is based on the case's Japan first information Aware Date (in PMDA > General tab of the Case Form). If this is a follow-up case, the Aware (latest significant follow-up) Date.
-
The Serious events must be Related events to be included in the list.
-
An event which is included in the past J-DSUR report is included in the Cumulative Total section of the count in the report.
-
For identifying the Japanese license, the license authorization country in the license configuration in Console is used. For the count of the foreign data, all configured products that have the same ingredients (regardless of the existence of Japanese license in this family) is considered as the target.
-
When the End Date is set as past date, any updates/new data that meet above condition in between the End Date and the current date are not included in this line listings. The data is the latest in the given timeframe, and not between the investigational timeframe Start Date and the Report Generation Date. This is achieved when you have enabled DLP and has configured the Use DLP Case Version radio option for J-DSUR configuration.
-
If there is 0 count on any counting numbering sections of the Line Listing, - is printed.
-
From 2nd and subsequent pages, the table header rows are repeated at the top of the each page.
Basic Data Retrieval Rule:
Cumulative Total:
-
In J-DSUR, the Cumulative Total is the count of Serious AEs that are collected between the Assigned Date and the end of the current investigation timeframe.
Investigation Time Frame
The Serious AEs from the cases collected in this investigation timeframe from among the cases retrieved by Cumulative Total.
4.2.8.4.1 Domestic Serious AE, etc. Case Occurence Status Line Listing
| Field Name | Description |
|---|---|
| Serious AE, etc. Case Occurrence Status Line Listing | This field represents the title of the paper form. |
| Attached paper form | This field represents the header of the paper form. |
| Information Source | This field represents the title of the row. |
| Clinical Study | This field represents the title of the column. This column has data only from the report source: Clinical study and Incident Country = Japan. It only considers the cases which contain the studies configured as Selected Studies in the Product Selection tab. |
| Investigation Time Frame | This field represents the title of the row. |
| This investigation period | This field represents the title of the column. This column collects count information in this investigation period only. |
| Cumulative Total | This field represents the title of the column. This column collects total number of count information in the entire reporting period starting from the first Assigned Date to the end of current investigation period.If the configuration is set (checked for Exclude the event count from the cumulative total if the event doesn't meet the condition), events that were reported in the past but as the condition has been modified in the current timeframe they no longer meet the condition, are removed. |
| This investigation period | This field represents the title of the column. This column collects count information in this investigation period only. |
| Cumulative Total | This field represents the title of the column. This column collects the total number of count information in the entire reporting period starting from the first Assigned Date to the end of current investigation period. |
| This investigation period | This field represents the title of the column. This column collects count information in this investigation period only. |
| Cumulative Total | This field represents the title of the column. This column collects the total number of count information in the entire reporting period starting from the first Assigned Date to the end of current investigation period. |
| Number of subjects | This information is captured from the J-DSUR tab. |
| AE, etc Case classification | This field represents the title of the column. |
| AE, etc. Case Count | This field represents the title of the column. |
| SOC name in Japanese | The SOC count is the count of cases (not AEs). The number is collected for this inv. Period and the total of entire J-DSUR time. |
| PT names | All the serious AEs are counted and entered for this inv. Period and entire J-DSUR time. |
| This investigation time frame: YYYY/MM/DD | This investigation timeframe is entered using the Japanese date format. |
| Terms for AE: MedDRA/J version ( ) was used. | The used MedDRA/J version must be entered in the brackets. |
4.2.9 Seiyakukyo Line Listing Report (Individual Report Common Line List)
Seiyakukyo Line Listing report is a periodic report format specified by the JPMA. This report is to be used by pharmaceutical companies in Japan to send case reports to medical institutions. It is mandated by PMDA to report study cases to medical institutions and is required to be fully compliant in Japan.Seiyakukyo periodic report is handled in the similar manner as the other Japanese periodic reports - PSR/ReSD, and CSPSR. At a high level, it has a similar Configuration screen, similar Generation, Publishing, and Handling process as other Japanese Periodic reports. However, the report output format and data is different so as to match the Seiyakukyo format.
All the following date fields in the Seiyakukyo Line Listing main report output are printed in the "YYYY/MM/DD" format:
-
Dose Start Date and Dose Stop Date
-
AE Onset Date
-
Subject Timeframe
-
Report Submission Date
-
Japan Information Receipt Date
For example, full dates are printed as "2013/10/17" and partial dates are printed as "2013/10" or "2013".
This date format is applicable in Microsoft Word as well as CSV format report outputs.
4.2.9.1 Configuration Screen
-
A new menu item - Individual Report Common Line List is added in Console > Groups > Menus section for the Argus Groups for Seiyakukyo report.
-
It is added under Reports > Periodic Reports right after J-DSUR menu option.
-
It is marked enabled by default for Administrator Group.
-
It is marked enabled by default during New Argus group creation.
-
It is only displayed if Console > System Management > Enabled Modules > Japanese module is enabled.
-
The following options are available
-
Include listed and unlisted events
-
Include unlisted events only (default option)
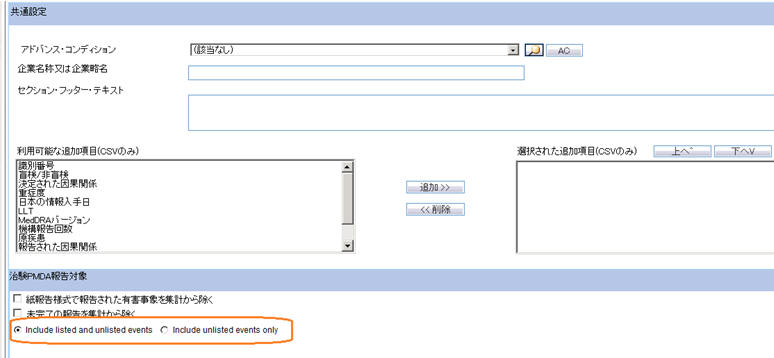
Description of the illustration ''fig7.jpg''
-
-
-
A new menu item - Individual Report Common Line List is added under Argus Safety > Reports > Periodic Reports menu.
-
It is added under Reports > Periodic Reports right after the CSPSR menu option.
-
This menu item is only available in Argus Safety if following conditions are met:
Console > Access Management > Users > User Type of the current user is configured as ARGUS J USER.
Console > System Management > Enabled Modules > Japanese module is enabled.
Console > Access Management > Groups > Menus section for Argus Groups has Reports > Individual Report Common Line List marked Enabled for at least one user groups for the current user.
-
-
Clicking on this menu in Argus Safety displays the existing Periodic Reports Library screen, which lists all the configured Seiyakukyo reports available in the system.
-
The functionality of this screen remains the same as existing except for the following:
The page breadcrumb for the Periodic Reports Library screen for Seiyakukyo is displayed as:
Reports > Periodic Reports > Serious, Unlisted AE Individual Case Communication Line ListThe screen name for this screen is displayed as Serious, Unlisted AE Individual Case Communication Line List.
When you click the Open button for a submitted Seiyakukyo report whose configurable becomes non-editable, the following warning message is displayed:
This configuration is not modifiable because the status of this Individual Case Communication Line List is Submitted
-
-
Clicking New or Open buttons on Periodic Report Library screen for Seiyakukyo reports displays the Configuration screen for Seiyakukyo reports which has 5 tabs similar to the CSPSR Configuration screen with following names. The Page Title text is: Individual Report Common Line List.
-
Subject of Report
-
Individual Report Common Line List
-
Scheduling
-
Security
-
-
The Subject of the Report tab is the same as the existing Subject of the Report tab for CSPSR.
-
The Study Selection tab has the following functionality:
Field Name Description Filter This button is available adjacent to the Available Clinical Compound Numbers textbox to allow filtering the data in the Available Clinical Compound Numbers multi-select list box.When you click this button, the data is refreshed in the Available Clinical Compound Numbers multi-select list box based on the search text specified by you in the textbox.Like search is performed based on the text specified by you. For example, If you have specified ABC as the search text, it performs case-insensitive text for %ABC%.The maximum allowed length of the Filter textbox is 70 characters. Available Clinical Compound Numbers It lists distinct Clinical Compound Numbers for all the licenses which are configured in the Console application. Selected Clinical Compound Numbers This listbox is populated based on your selection and removal of Clinical Compound Numbers by using Add and Remove buttons from/to the Available Clinical Compounds listbox. This is a mandatory field. If it is not specified, and the OK button is clicked, the following error message is displayed:
Clinical Compound Number is not selectedAdd This field allows you to move the selected item(s) from left hand listbox to the right hand listbox. Add All This field allows you to move all the items from the left hand listbox to the right hand listbox. Remove All This field allows you to move all the items from the right hand listbox to the left hand listbox. Remove This field allows you to move the selected item(s) from the right hand listbox to the left hand listbox. Classifications for Clinical and Domestic Study Cases It lists all the Case Classification (J) values from the Console code list. If J is not available, the corresponding English value is displayed. You can select multiple values in this listbox by using Ctrl + Click and Shift + Click combinations.
The first option in this list is always displayed as: All Case Classifications
These case classifications are used for fetching the case data for Clinical and Domestic study cases reporting in Seiyakukyo report.
Classifications for Foreign "Ichihen" Study Cases It lists all the Case Classification (J) value from the Console code list. If J is not available, the corresponding English value is displayed. You can select multiple values in this listbox by using Ctrl + Click and Shift + Click combinations.
The first option in this list is always displayed as: All Case Classifications
These case classifications are used for fetching the case data for Foreign Ichihen study cases reporting in Seiyakukyo report.
-
Seiyakukyo Report tab has the following functionality:
# Field Name Description 1 Individual Report Common Line List N/A 2 Common Configuration N/A 3 Company Name or Company abbreviation You can specify the value of the Company Name to be printed in the second column of Seiyakukyo report using this field. The maximum allowed length of this textbox is 60 characters.
It allows English as well as Japanese characters.
4 Section Footer Text You can specify a text to be printed as footer text at the end of each section in the report output using this field. The maximum allowed length for this textbox is 200 characters.
It allows English as well as Japanese characters.
5 Available Additional Columns (CSV Only) This field lists all the available additional fields that can be added to the Seiyakukyo report CSV output. These are not applicable to the Word output. The following fields are available in this list in the alphabetical order:
ACK Number
Blinded / Not Blinded
Determined Causality
Event Intensity
Japan Information Receipt Date
LLT
MedDRA Version
Number of times this report is submitted to PMDA
Primary Disease
Reported Causality
Report Submission Date
SOC
Study Name
6 Selected Additional Columns (CSV Only) It lists all the selected additional fields that are added to the Seiyakukyo report CSV output. These are not applicable to the Word output. You can move items between Available and Selected Additional Columns using the Add and Remove buttons.
While adding items, these items are listed in the same order in which they are added.
You can move items up and down using Up and Down buttons.
The Seiyakukyo report output includes these columns at the end in the same order as specified in this list.
7 Add This field allows you to move the selected item(s) from the left hand listbox to the right hand listbox. 8 Remove This field allows you to move the selected item(s) from the right hand listbox to the left hand listbox. 9 Up ^ This field allows you to move the selected item in the Selected Additional Columns, one position closer to top of the list. 10 Down V This field allows you to move the selected item in the Selected Additional Columns, one position closer to the bottom of the list. 11 Clinical and Domestic Study Cases N/A 12 Exclude events which were reported by paper report forms If this checkbox is checked, all the events which are reported through paper reports are excluded. This logic is applicable only for Clinical and Domestic Study cases for which events are reported based on Expedited/E2B reports.
It is unchecked by default.
13 Exclude Incompletion reports If this checkbox is checked, all the events which are reported through Expedited/E2B reports where the value of J.6 (Mhlwadmicsrcompleteclass) = 1 (Incomplete) are excluded. This logic is applicable only for Clinical and Domestic Study cases for which events are reported based on Expedited/E2B reports.
It is unchecked by default.
14 Include listed and unlisted events (new) You can specify the criteria for printing the events under Regular and Domestic Study cases category using this field. This radio button is selected by default if the "Include only serious unlisted event" checkbox was not selected for the already configured reports.
15 Include unlisted events only (new) You can specify the criteria for printing the events under Regular and Domestic Study cases category using this field. This radio button is selected by default:
-
For all new reports.
-
If the "Include only serious unlisted event" checkbox was selected for the already configured reports.
16 Foreign "Ichihen" Study Cases N/A 17 Include foreign AE marked as "Not include for the report in Japan" If this checkbox is checked, all the events in the cases which are marked as Not to be reported to Japan in the Case Form Events tab are also included. This logic is applicable only for Foreign Ichihen study cases for which events are reported based on case data directly.
It is unchecked by default.
18 Append CIOMS reports If this checkbox is checked, the CIOMS reports are created and added at the end of Seiyakukyo report output for all the cases which are printed under Foreign Ichihen Study Cases section.These CIOMS report are printed for the earliest Valid Japanese license for the first suspected product in the case. If the first suspected product does not have a Valid Japanese license, the next suspected product which satisfies this criterion is picked-up in the same order in which they are present in the case. Once the Seiyakukyo is generated as FINAL, these reports are listed in the Case under the Regulatory Reports tab in the similar manner as for English PSUR.These reports are added only in the PDF format (not in CSV), when the Seiyakukyo report is published.These reports are generated at the time of Seiyakukyo generation. The Generation Status dialog box displays the label Generating CIOMS reports for this task during the Seiyakukyo generation process. Although, generated at this stage, these CIOMS reports are visible to users after the Word format is published into PDF format.Even if you modify the Events/Cases printed in the application generated Word/CSV formats of Seiyakukyo report, the application still prints the CIOMS reports based on the application generated case list under Foreign Ichihen Study Cases section.It is unchecked by default. 19 DLP support It displays 2 options to allow support of DLP for fetching case data for Foreign Ichihen study case: Use Current Case Version and Use DLP Case Version. This radio button control is only displayed if DLP is enabled.
Based on the PSR/CSPSR configuration for DLP, the report is executed accordingly using the latest Case Revision or using the DLP Case Revision.
Use Current Case Version is always checked by default.
-
-
The Scheduling tab is the same as the existing Scheduling tab for CSPSR, except for the following:
-
The following date fields are not displayed as they are not applicable to the Seiyakukyo reports. All the validation checks related to these fields are also not applicable to the Seiyakukyo reports:
Assigned Date
Clinical Study Plan Submit Date
Clinical Trial for Partial Change Plan Submit Date
-
The Start Date of first timeframe is editable as long as this timeframe is not submitted. However, the Start Date of second timeframes and onwards keep following the existing logic of being disabled and pre-populated as next day of the previous timeframe End Date.
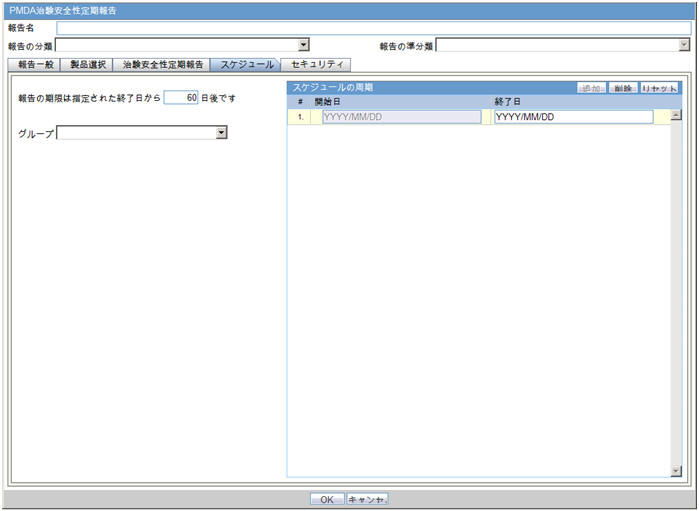
-
-
The Security tab is the same as the existing Scheduling tab for CSPSR:
4.2.9.2 Report Output Format
-
This report prints information from two types of study cases:
-
Clinical and Domestic Study Cases
-
Foreign Ichihen Study Cases
-
-
The report output prints the events from these 2 types as 2 separate sections starting from new pages.
-
Within each of these 2 types, the report output prints the events for each of the configured Clinical Compound Numbers starting from new pages as well.
4.2.9.3 Report Output Filter Criterion
-
Regular (Clinical) and Domestic Study Cases - All the data is fetched based on the events reported in the Expedited/E2B reports already reported to PMDA.
-
Only the Expedited/E2B reports sent to the reporting destinations which are configured as the Seiyakukyo Configuration > Subject of Report tab > Primary Agency are considered.
-
Only the Expedited/E2B reports whose cases have at least one of their Case Classifications configured as Seiyakukyo Configuration > Study Selection tab > Selected Classifications for Regular and Domestic Study Cases are considered.
-
Only the Expedited/E2B reports that have J.11 (mhlwcompoundnum) element value same as one of the values configured as Seiyakukyo Configuration > Study Selection tab > Selected Clinical Compound Numbers are considered.
-
Only the Expedited/E2B reports that have J.4a (mhlwadmicsrcasenumclass) element value as 8, 9, 10, and 11 (reporting categories - H, I, J, and K) are considered.
-
All Nullification and Downgrade reports are ignored. However, their Initial and Follow-up reports that fall within the Seiyakukyo current timeframe are still considered.
-
If multiple Initial or Follow-up Expedited/E2B reports for any product license (any series of Expedited/E2B reports) fall within the current timeframe, all of them are considered.
-
Only the Expedited/E2B reports that have Report Submission Date (GMT) between and including the Start and End date (after conversion to GMT based on database time zone offset) configured for the current timeframe of the Seiyakukyo report are considered.
-
If the radio option to include Unlisted events only is configures, it includes only those reported events from the Expedited/E2B reports that are unlisted in the case.
Unlisted - Specified as Unlisted in the case against atleast one Japanese Investigational license (License Drug Authorization Country as Japan) having same clinical compound number as configured in Seiyakukyo report. The Listedness specified as Unknown is not considered.
-
If the radio option to include Listed and Unlisted events is configures, it includes all the reported events (listed and unlisted both).
-
If the checkbox to exclude incompletion reports is checked on the Individual Report Common Line List configuration tab, any Expedited/E2B report that have J.6 (Mhlwadmicsrcompleteclass) element value as 1 (Incomplete) is ignored.
-
If the checkbox to exclude Paper reports is checked on Individual Report Common Line List Configuration tab, all PMDA Paper Expedited reports are ignored.
-
If the same AE/Infection event is reported in multiple eligible reports from the same case, all of them are reported multiple times in the Seiyakukyo report as well.
-
All the events in Seiyakukyo report for Regular (Clinical) and Domestic Study Cases are sorted in the following order:
-
Case ID Number (ascending)
-
Report Submission Date (Old to New)
-
Events as ordered in the expedited/E2B report
-
-
-
Foreign Ichihen Study Cases - All the data is fetched based on the events present in the case data.
-
Only the cases that have Country of Incidence other than Japan are considered.
-
Only the cases that have at least one of their Case Classifications configured as Seiyakukyo Configuration > Study Selection tab > Selected Classifications for Foreign Ichihen Study Cases are considered.
-
Only the cases that have atleast one of their Japan Information Receipt Date (significant follow-up or initial receipt date) between and including the Start and End date configured for the current timeframe of the Seiyakukyo report are considered.
-
Only the cases that have one of their Japanese licenses as present in the Case Form > Event Assessment tab and have the same Clinical Compound Number configured as Seiyakukyo configuration > Study Selection tab > Selected Clinical Compound Numbers are considered.
-
Only those events from the cases that have the Seiyakukyo products specified as:
Related - At least one of the As Reported and As Determined Causality in the case is specified as Reportable for this Product Event combination.
Unlisted - Specified as Unlisted in the case against at least one Japanese Marketed or Investigational license (License Drug Authorization Country as Japan) having same clinical compound number as configured in Seiyakukyo report. The Listedness specified as Unknown is not considered.
Serious - The event is specified as Serious.
-
All the events in the Seiyakukyo report for Foreign Ichihen Study Cases are sorted in the following order:
-
Case ID Number (ascending)
-
Japan Information Receipt Date (significant follow-up or Initial Receipt Date) (Old to New)
-
Events as ordered in the case
-
-
-
If the checkbox to include foreign AE marked as Not include for the report in Japan is checked on the Individual Report Common Line List Configuration tab, those events are also included. Else, such events are ignored from the cases.
4.2.9.4 Report Output Field Mappings
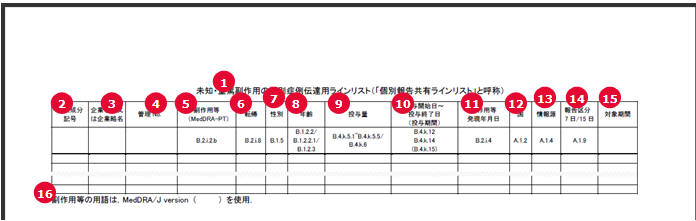
| # |
Field Name | Description |
|---|---|---|
| 1 | Serious, Unlisted AE Individual Case Communication Line List | This represents the title of the paper form.
It is a fixed text to be printed in the top center of the Report Output table. |
| 2 | Clinical Compound Number | Clinical Compound Number of the report/case.
For Clinical and Domestic studies, it prints J.11 (mhlwcompoundnum) element value from the expedited/E2B report. For Foreign Ichihen studies, it prints the Clinical Compound Number from the License Configuration for the Japanese licenses present in Case Form > Event Assessment tab. |
| 3 | Company name or Company name abbreviation | If you have specified the Company Name to be printed in the Individual Report Common Line List configuration tab, that text is printed as it is for all the event rows. Else, it is left blank. |
| 4 | Management No. (Case No) | The Argus Case ID number for the corresponding event is printed in this field. |
| 5 | Adverse Event (MedDRA-PT) | This field represents the name of the Serious and Unlisted MedDRA J Term (B.2.1.2.b)
For clinical and domestic studies: It prints MedDRA PT (J) term based on the B.2.1.2.b (REACTIONMEDDRAPT) value from the Expedited/E2B report. For decoding the MedDRA PT code from the Expedited/E2B report into the J Term, latest MedDRA dictionary configured for event encoding is used. For foreign ichihen studies: It prints MedDRA PT (J) term from Case Event record. |
| 6 | Outcome | This field represents the Reaction Outcome (B.2.i.8)
For clinical and domestic studies: It prints the following Japanese description text for the E2B code values from the B.2.i.8 (REACTIONOUTCOME) value from the Expedited/E2B report. For foreign ichihen studies: It prints the Event Outcome (J) value from the Case Event record. If no value is available in the case, then print NULL. The Reaction Outcome footer in PSR Form 4 has E2B R3 code for Reaction Outcome against E2B R2 Code 6. |
| 7 | Gender | This field represents the Patient Gender (B.1.5).
For clinical and domestic studies: It prints the E2B M2 Japanese description based on the B.1.5 (PATIENTSEX) value from the Expedited/E2B report or the case. If B.1.5 is not available, print Unknown For reporting categories L, M, and N, it is left blank. For foreign ichihen studies: It prints the Patient Gender (J) value from the Case Patient record. If no value is available in the case, print Unknown |
| 8 | Age | This field represents the Patient Age (B.1.2.2/B.1.2.2.1/B.1.2.3)
It prints the Patient Age value based on the E2B logic for Patient Age elements. For example, If the Child Only checkbox in Case is checked, only GESTATIONPERIOD (B.1.2.2.1) is transmitted. If it is not checked, and if PATIENTONSETAGE (B.1.2.2) is specified, only it is transmitted else PATIENTAGEGROUP (B.1.2.3) is transmitted. For PATIENTONSETAGE, age (B.1.2.2a) value is printed followed by the E2B M2 Japanese description of the Age Unit (B.1.2.2b) without any space in between . For PATIENTAGEGROUP, E2B M2 Japanese description of B.1.2.3 is printed. For GESTATIONPERIOD, TBD is printed as fixed text followed by the Gestation period (B.1.2.2.1a) value and the E2B M2 Japanese description of the unit (B.1.2.2.1b) without any space in between. For PATIENTONSETAGE, if the unit value is Decade (B.1.2.2b = 800), special printing rule is followed. |
| 9 | Dose | This field represents the Product Dose Information (B.4.k.5.1~B.4.k.5.5/B.4.k.6)
In case of multiple dosages for the Seiyakukyo product(s), all the dosages are printed in the same cell, in the same order as in the Expedited/E2B report or as per the Product and Dosages order in the case. Each new dose information is printed after leaving a blank line and in alignment with the corresponding dose Start/Stop date value which is printed in the next column. For Clinical and Domestic studies, each dose information is printed using the following format: [B.4.k.5.1][B.4.k.5.2 (using E2B M2 Japanese description)][B.4.k.5.3]?/[B.4.k.5.4][B.4.k.5.5(using E2B M2 Japanese description)] - TBD([B.4.k.6(If this does not exist, then its braces for this value are not printed. However, a blank line is left to align the Dosage Information with the corresponding dosage dates values in the adjacent column.)]) For foreign ichihen studies, each Dose Information is printed in the following format using the value directly from the Case Product Dosage Record: [Dose][Dose Unit (J)]Code List/Dosage Frequency/[Number]TBD/[Unit Number][Unit (J)]([Dose Description (J)]) - If this does not exist, then its braces for this value is not printed. However, a blank line is left to align the Dosage Information with the corresponding dosage dates values in the adjacent column. |
| 10 | Dose Start Date ~ Dose Stop Date (Dose interval) | This field represents the Product Dosage Start/Stop DatesB.4.k.12B.4.k.14(B.4.k.15)
In case of multiple dosages for the product(s), all the dosage dates are printed in the same cell, in the same order as in the Expedited/E2B report or in the case. Each new Dosage Date is printed after leaving a blank like and in alignment with the corresponding dosage information value which is printed in the previous column. For foreign ichihen studies, each dosage date is printed using the Dose Start Date, Dose End Date, and Dose Duration values from the Case Product Dosage record in the same format as specified for Clinical and Domestic studies. Dose duration value is converted from seconds into appropriate unit using the same logic as used in E2B for B.4.k.15. |
| 11 | AE, etc. onset date | This field represents the Reaction Onset Date (B.2.i.4)
For Clinical and Domestic studies, it prints the REACTIONSTARTDATE (B.2.i.4a and B.2.i.4b) value. For foreign ichihen studies, it prints the Event Onset Date from Case Event Record. |
| 12 | Country | This field represents the Country of Incidence (A.1.2)
For Clinical and Domestic studies, it prints the E2B M2 Japanese description based on the A.1.2 (OCCURCOUNTRY) value from the Expedited/E2B report. For foreign ichihen studies, it prints the Country Name (J) value from the Case Record. |
| 13 | Information Source | This field represents the Report Type (A.1.4)
For clinical and domestic studies, it prints the E2B M2 Japanese description based on the A.1.4 (REPORTTYPE) value from the Expedited/E2B report. For foreign ichihen studies, it prints the Report Type (J) value from the Case Record. |
| 14 | Report Type7 days/15 days | This field represents the meeting Expedited Report Criteria (A.1.9)
For clinical and domestic studies, it prints 7 days or 15 days, if the value of A.1.9 (FULFILLEXPEDITECRITERIA) is 1 or 2 respectively, in the Expedited/E2B report. For Foreign Ichihen studies, it is left blank. |
| 15 | Subject timeframe | This field represents the Reporting Timeframe considered for the AE.
It prints the Seiyakukyo report current timeframe. The same values are printed in all the rows. |
| 16 | For the AE terms, MedDRA/ J version ( ) is used. | This field represents the MedDRA J version used for this report.
It prints the MedDRA version as currently configured in the application for event encoding. This is repeated at the end of each Clinical Compound Number section. |
| 17 | Any additional columns added by the user in configuration (applicable only to CSV output) | Study Name:
For clinical and domestic studies, it prints the value of A.2.3.2 (SPONSORSTUDYNUMB) from the Expedited/E2B reports. For foreign ichihen studies, it prints Study ID (J) from the case record. If Study Name (J) is not available in the case, print the corresponding English value. Blinded / Not Blinded: For all types of cases, it prints the Japanese text - Blinded or Not Blinded, based on the Case Form > Study Information > Study Type field value for the corresponding case. SOC: For clinical and domestic studies, it prints the MedDRA SOC (J) Term based on the B.2.1.2.b (REACTIONMEDDRAPT) value from the Expedited/E2B report. For decoding the MedDRA PT code from the Expedited/E2B report into J Term, latest MedDRA dictionary configured for Event Encoding is used.For foreign ichihen studies, it prints the MedDRA SOC (J) Term from the Case Event record. NOTE: Since L, M, and N category cases are not included in the report, this data will no longer be left blank for these categories. LLT: For clinical and domestic studies, it prints the MedDRA LLT (J) Term based on the B.2.1.2.b (REACTIONMEDDRALLT) value from the Expedited/E2B report. For decoding the MedDRA PT code from the Expedited/E2B report into J Term, latest MedDRA dictionary configured for event encoding is used.For foreign ichihen studies, it prints the MedDRA LLT (J) Term from the Case Event record. NOTE: Since L, M, and N category cases are not included in the report, this data will no longer be left blank for these categories. MedDRA Version:For clinical and domestic studies, it prints the MedDRA version as used in B.2.i.2.a (REACTIONMEDDRAVERSIONPT) from the Expedited/E2B report.For foreign ichihen studies, it prints the MedDRA version from the case for that Event Encoding. NOTE: Since L, M, and N category cases are not included in the report, this data will no longer be left blank for these categories. |
| Report Submission Date: For Clinical and Domestic studies, it prints the corresponding Expedited/E2B Report Submission date after converting it into Japanese timezone based on the Argus J > Reporting > Offset from GMT Common Profile Switch that is used to calculate Japanese date/time fields for Interchange-J (in hours). For Foreign Ichihen studies, it is left blank.
Japan Information Receipt Date: For Clinical and Domestic studies, it prints J.3b (mhlwadmicsrinfoobtndatesource). For Foreign Ichihen studies, it prints the Japan Aware date. ACK Number: For Clinical and Domestic studies, it prints the eight digit PMDA ACK number received for the corresponding Expedited/E2B report. For Foreign Ichihen studies, it is left blank. Number of times this report is submitted to MHLW: For clinical and domestic studies, it prints J.5 (mhlwadmicsrmhlwcumreporttimes) element value. For Foreign Ichihen studies, it is left blank. Primary Disease: For all types of cases, it prints the Condition (J) value as present in Case Form > Patient tab > Other Relevant History section. Only those items are printed which have Primary Disease text present in the Notes (J) field. In case multiple items have to be printed, then these are printed in separate lines in the same cell. Event Intensity: For all types of cases, it prints the Case Form > Event tab > Intensity (J) field value. NOTE: Since L, M, and N category cases are not included in the report, this data will no longer be left blank for these categories. |
||
| Reported Causality:
Only one value is printed for this field based on the Reported Causality values specified for those products in the case, whose valid Japanese licenses have the same Clinical Compound Number for which this event is being printed. Only the causality values that are related to the current event being printed are considered. The following rule is used to determine the value that is printed in the report output. Related - If any of the Reported Causalities is Reportable (Consider Expeditable to Authorities is checked in Console Code list) Not related - Else, if any of the Reported Causalities excluding Unknown is not reportable Unknown - Else, if any of the Reported Causality is Unknown No value - Else, if all the Reported Causalities are blank. NOTE: Since L, M, and N category cases are not included in the report, this data will no longer be left blank for these categories. Determined Causality: Only one value is printed this for field based on the Determined Causality values specified for those products in the case, whose valid Japanese licenses have the same Clinical Compound Number for which this event is being printed. Only the causality values that are related the current event being printed are considered. The following rule is used to determine the value that is printed in the report output. Related - If any of the determined causalities is Reportable (Consider Expeditable to Authorities is checked in Console Code list) Not Related - If any of the determined causalities is Reportable (Consider Expeditable to Authorities is checked in Console Code list) Unknown - Else, if any of the determined causalities excluding Unknown is not Reportable No Value - Else, if all the determined causalities are blank. NOTE: Since L, M, and N category cases are not included in the report, this data will no longer be left blank for these categories. |
||
| 18 | Section Footer Text | It prints the configured text as specified in Individual Report Common Line List Configuration tab > Section Footer text below the event output table right after MedDRA J Version, in a new line.
This text is repeated at the end of each Clinical Compound Number section. |
| 19 | Page Numbering | The page numbering for the Seiyakukyo report starts from 1 for each Clinical Compound Number section independently.
The page numbering for Seiyakukyo report is printed in the middle of page footer in the following format: <current page number>/<Total Pages> The page numbering for attached CIOMS reports starts from 1 for each CIOMS independently. The page numbering for attached CIOMS reports is printed in the top left corner of CIOMS report table (same as in attached CIOMS in PSURs) in the following format: <current page number>/<Total Pages> |
| 20 | Page Size/Font | The MS Word A4 Landscape page size is used for Seiyakukyo report.
Ms Mincho font is used for the report output. |
4.2.9.5 CSV Output Format
The Seiyakukyo report also supports printing in the CSV format.
-
The Seiyakukyo configuration data is not printed in the CSV format. Only the main Seiyakukyo report output is printed in CSV format.
-
The Report Title is put as the first value in the first row.
-
All the event rows from the report output including the Header row are put in separate rows.
-
The MedDRA J version and Section Footer Text are put as separate rows at the end, in the same way as the report output.
-
Each cell value for a row is put as individual item within that row in the CSV format.
-
All the comma characters and new line characters within a cell value are supported and maintained in the CSV format. It must not break that cell value into multiple items in CSV. This is achieved by using double quote characters for the cell values to escape such characters.
-
All the double quote characters within a cell value are replaced by 2 double-quote characters in the CSV format.
-
All valid Japanese characters as specified for the PMDA E2B file (Main ESM J SRS > 19.6.1 point # 15 (c)) are supported by this CSV format.
-
Different sections related to different case types and Clinical Compound Numbers are separated by a single blank line.
4.2.9.6 Periodic Report Process Flow
The Seiyakukyo Periodic report is handled in the same manner as other Japanese Periodic reports - PSR/ReSD and CSPSR. All the actions that are applicable to the existing Japanese reports are supported for Seiyakukyo Periodic reports as well.
-
All the configured Seiyakukyo reports are listed on the Periodic Report Configuration page.
-
All the actions supported on the Periodic Report Configuration page for CSPSR are supported for Seiyakukyo reports as well.
-
New Report
-
Copy
-
Modify
-
Delete
-
Print
-
-
The Print dialog box for Seiyakukyo lists 2 output formats in the drop-down adjacent to the Run Now option - Word and CSV. The Word output format is selected by default.
-
Choosing the Word format for the Run Now action and clicking the OK button displays the output of the Seiyakukyo reports in Word format in the similar manner as CSPSR.
-
Choosing CSV format for Run Now action and clicking the OK button displays the Seiyakukyo reports in CSV format.
-
Irrespective of the format - Word or CSV, if the report is executed as Final or Draft, it is available in the same format through the standard - FINAL and DRAFT links in the row for that report on the Periodic Report Library screen. In case, you have executed the report with Internal and other options, it is available in the same format using the DRAFT link on the Periodic Repot Library screen.
-
No Watermark is put on the CSV format output. It is applicable only for the Word format output in the similar manner as CSPSR.
-
If a Submitted report is attempted to be printed in the CSV format, the application displays the following error message with the OK button:
The report is already submitted. You cannot print this report again in CSV format.
However, for Word format, it prints the Configuration page only without any error message.
-
-
All the Seiyakukyo reports are also listed on the Reports > Compliance > Periodic Reports screen.
-
All the actions supported on Reports > Compliance > Periodic Reports screen for CSPSR are supported for Seiyakukyo reports as well.
-
The View and Edit Seiyakukyo dialog box (View Report right-click menu option) from Reports > Compliance > Periodic Reports screen, supports all the actions (in same manner as View and edit J-DSUR dialog box for CSPSR) for Seiyakukyo Word as well as CSV formats.
-
View
-
Check-in
-
Check-out
-
Publish
-
-
The View or Check-out option displays the Seiyakukyo report in the same format (Word or CSV) that is chosen/checked-in by you.
-
You are allowed to check-out the report in one format (CSV) and check-in the file in other format (WORD).
-
If you click the Publish button and the last checked-in file is in the CSV format, it displays the following warning message with Yes and No buttons:
As this report exists in CSV format, it will not be converted into PDF format while publishing it. It will be published as it is in CSV format itself. Do you want to proceed?
4.3 Non-Reportable and Invalid Events
When all the event in a case become Non-Reportable then system will scheulde a Downgrade report.
When all the events in the case becomes Invalid then system will schedule a Nuliffication report for Marketed Licenses(Reporting Catgeory: 1,2,3,4).
When all the events from the previously submitted Report becomes Invalid then system will schedule a Nuliffication report for Investigational Licenses(Reporting Catgeory:8,9,10,11).