Site portal
We updated study close message behavior, Site portal users can now invalidate the most recent version of a document in the site's Library documents tab, the Feasibility survey version history drawer’s subtext now clarifies available actions, and more.
Close study message
In this release, we updated the behavior of the study close message in the Site portal. Now, when a site completes all assigned workflow tasks and the workflow is closed, the site can manually close the displayed message.
After closing the message, if the user is logged in, they can select another study to work on, if available. They can also review previously submitted tasks, but cannot update them.
Note that the user cannot:
- change their response to a Verify site interest (and interest again) task
- complete any additional workflow tasks
- edit a submitted survey task
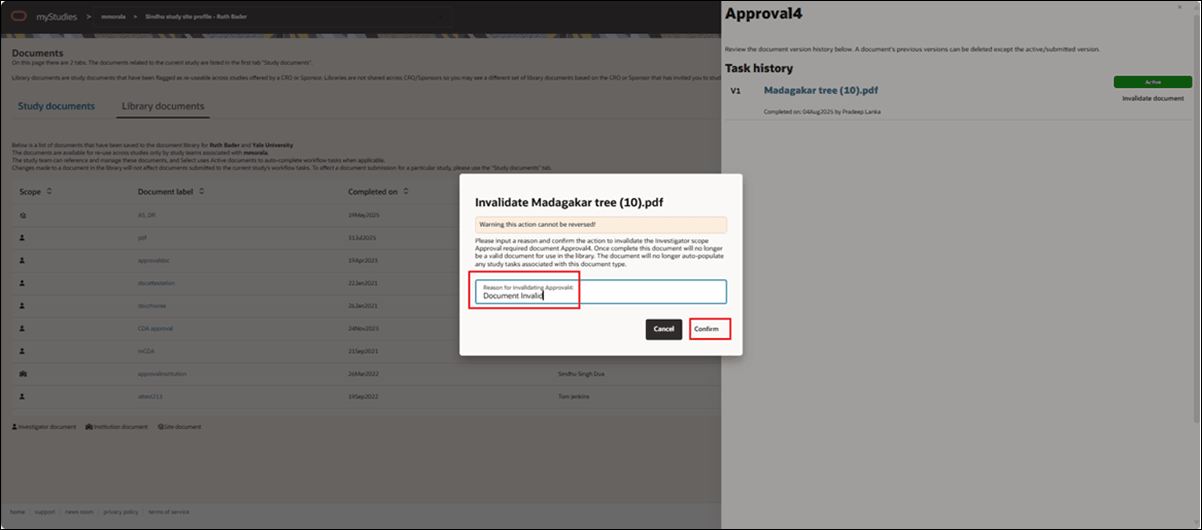
Invalidate a library document
With release 25.1.1, Site portal users can now invalidate the most recent version of a document in the site's Library documents tab (excluding "download only" document types). For an Active document, the users will click the new Invalidate document button, enter a required reason, and the document will be marked as invalid. Invalidation details (who performed the action, when, and why) are saved in the document history. When viewing the Document history grid, Site portal users can hover over the invalidated document icon to see the invalidation details or click the document name to open the document drawer for full details.
If an Oracle Site Select user later uploads a new version for the same document label, that version will become Active, and the details related to the previous invalidation will remain visible in the document history drawer.
As part of this update, we also renamed the Library Documents tab's Actions column to Response.
Generic Document responses and actions in Document Library
This enhancement updates the Site portal’s Documents page so users can view responses and perform actions for Attestation type Generic Documents on both the Study documents tab and the Document library tab. The Study documents tab will show the document, its history, and the response, while the Library document tab will show the same information and allow the site user to update the response saved in the document library.
When the site user views any Attestation type Generic document in the Document library (at the investigator, institution, or site level), they'll see the saved response option from the library’s metadata. If a reason for a response change was provided, a pencil icon displays with the change reason as hover text. If the document is invalidated, the chosen response is not displayed.
In the document’s history drawer in the “Response” section, the site user can download the active file as a link showing the file name. Task options are as editable radio buttons, with the displayed text and selected choices matching what is saved in the library. If a response was previously edited, the site user sees the previous change reason and the user name and date below the radio options. They'll have the ability to change the saved response; if they do, a confirmation dialog will asks them to confirm the new response and provide a reason (up to 2,000 characters, required), with "No" and "Yes" options to cancel or proceed. After saving the change, the history drawer refreshes to show the latest information.
For Attestation document types, we also updated the drawer’s top subtext on the Library tab. The new text is: “Review the library entry for this Attestation document type. The response saved to the library can be changed. The document can also be invalidated. Invalidated documents will no longer auto-populate study workflow tasks.”
Feasibility survey version history drawer
We updated the subtext shown at the top of the Feasibility survey version history drawer to clarify the actions available to the Site portal user. The revised text is, “Review the Feasibility survey submission version history below. Clicking on the survey name will download a PDF copy of the submitted responses.
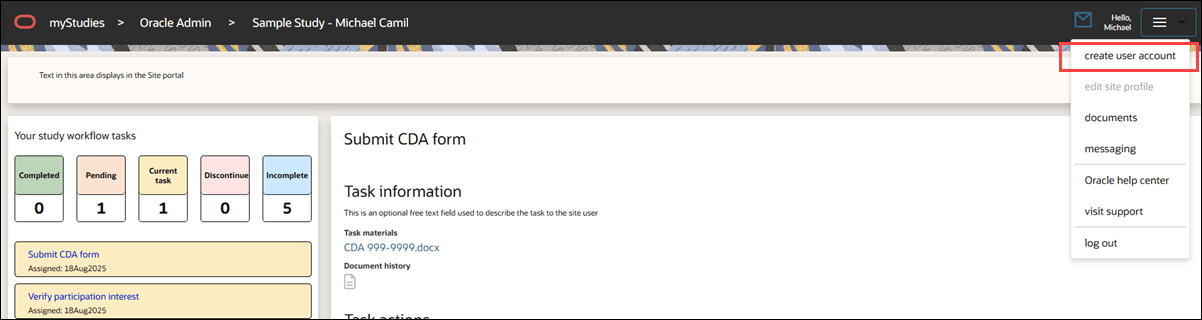
Create an account
In this release, we returned the ability for site users to create a Site portal account via the study invitation No login link. When accessing the portal this way, site users see a "Create user account" menu option instead of "Edit user profile." Clicking the account creation link prompts them to enter their email address, and they’ll receive a password reset email to set their account password.
By design, if you are an Oracle Site Select user and you click the No login link in the Invitation received modal, you will not be able to create or edit a Site user's login account.
Pending CDA helper text
Previously, Site portal users had to hover over the disabled Submit button to see why it was unavailable. Now, when a CDA task is in the Pending Review state, the message "Your document submission is currently pending review. There is no action to take." appears next to the disabled button, with no hovering required. This change improves usability and reduces confusion.
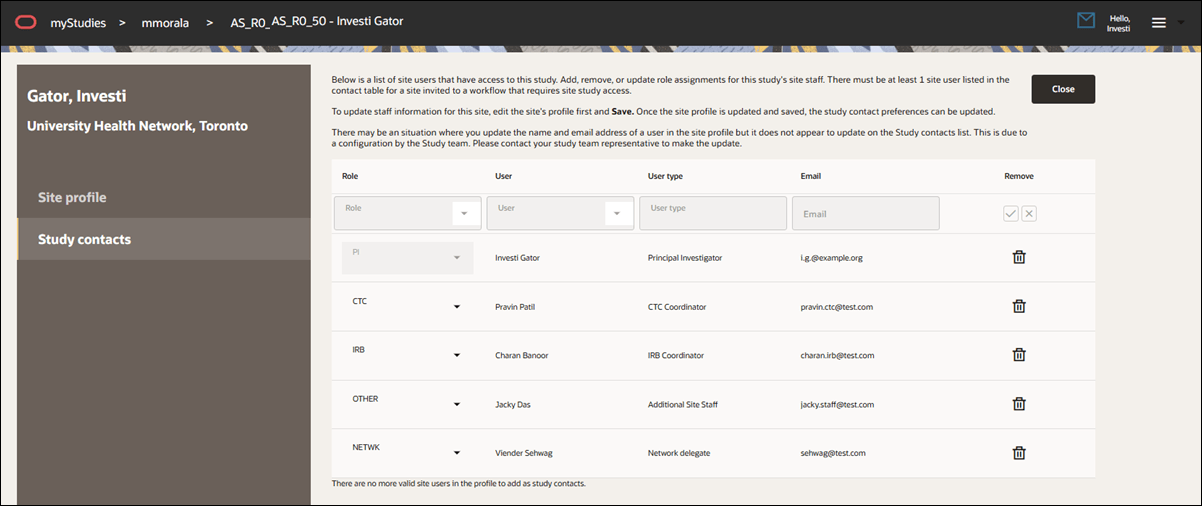
Study contacts list
In this release, we added Study contacts functionality to the Site profile. This functionality duplicates the feature as it exists in Oracle Site Select today.
Study contacts functionality allows Site portal users to manage site-level contact assignments specific to each study. The tab displays a table of study contacts that includes the role, user details, user type, email address, and a remove option. All data in this table is sourced from the corresponding site profile, and changes to user details must be made and saved in the site profile before they can be reflected here. If name or email updates are not reflected in the Study contacts list, this is likely due to datasource priority, and Site portal users are advised to reach out to their study team representative.
Roles include Investigator, Clinical Trial Coordinator (CTC), IRB contact, site staff, and network delegate. Users are identified by their full name and degree as defined in the site profile, and their user type is determined by the category they appear under in the site profile. If the study contact list includes only one user, the remove icon is disabled with a tool tip explaining that at least one contact is required. When additional site profile contacts are available, the remove option becomes active, including for the Principal Investigator. Users can add a contact by selecting a role and user from drop downs, which display only if eligible users are available to add. If all valid contacts have been added, the drop-downs will be disabled and a message will explain why.
The Study contacts table updates automatically once all required fields are completed. If a new site profile user is added, they will appear as a selectable option for Study contacts, and the message about unavailable users will disappear. Additionally, if a site is affiliated with a network, and the delegate is removed from the study contacts list, the delegate will not be re-selectable unless added again through the profile.
Any updates to user name or email made by site users or Oracle Site Select users in the site profile will carry over to the Study contacts list once the site profile is saved, but only if the site profile is the highest-priority datasource. If a PI is removed from the Study contacts list, that user will lose access to the study in the Site portal. All changes made on the Study contacts page are auto-saved, and the "Close" button returns users to the site grid.
Caution:
If the site profile is not set as the highest-priority study data source, updates to a user’s name or email in the site profile may not appear in the Study contacts list. This is expected behavior, but it may cause confusion for Site portal users.
Site portal user cheat sheet
With release 25.1.1, we updated the Site portal help document. Site portal users can click menu ![]() in the page header to open the PDF document using the "Oracle help center" link.
in the page header to open the PDF document using the "Oracle help center" link.
Parent topic: What's new