|
|
| HDR Version 7 HL7 Version 3 Messaging Conformance Specification |
| Contents Previous Next Conformance Examples |
This section contains the following topics:
5.12.1 Introduction 5.12.2 RMIM 5.12.3 Referenced CMETs 5.12.4 Links to Artifacts
The A_DrugIntervention (universal) CMET represents drug intervention information for individual case safety reporting. It captures the substance administration related information that is relevant for drug administrations possibly related to an adverse reaction. The relevant information includes items related to the substance administration, component parts of a more general administration act, as well as ancillary information related to the substance administration. This message element type is used to capture substance administrations for drugs, therapeutic biologics, and vaccines.
The following figure shows the A_DrugIntervention (universal) RMIM:
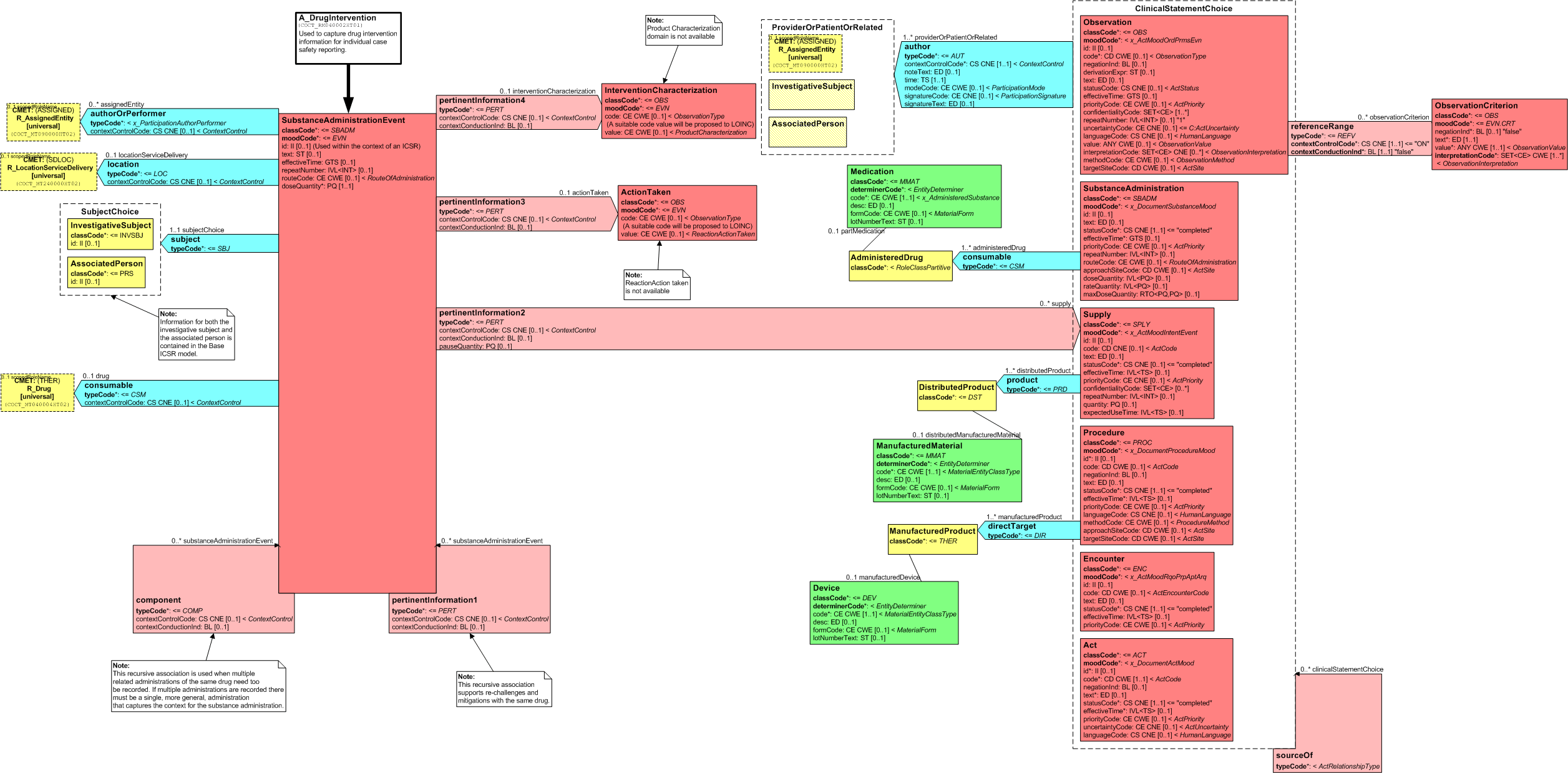
Figure: 5.12.2-1 A_DrugIntervention (universal) RMIM
| Artifacts | Link |
| Visio File | coct_rm040002ht01.vsd |
| Visio XML File | COCT_MT040002HT01.xml |
| Hierarchical Message Description (HMD) | |
| Schema | coct_mt040002ht01.xsd |
| Model Interchange Files (MIF) | coct_mt040002ht01.mif |
| Contents Previous Next Conformance Examples |
|
Copyright © 2018, Oracle. All rights reserved. |
About Oracle Contact Us Legal Notices and Terms for Use Privacy Statement |