|
|
| HDR Version 7 HL7 Version 3 Messaging Conformance Specification |
| Contents Previous Next Conformance Examples |
This section contains the following topics:
4.29.1 Introduction 4.29.2 RMIM 4.29.3 State Transitions/Trigger Events 4.29.4 Interactions 4.29.5 Referenced CMETs 4.29.6 Links to Artifacts
The Substance Administration Order RMIM represents a verified order placed by a certified provider for medications and other therapeutic consumable substances intended for use by a particular patient. Substance administration orders do not include orders related to supply of items not intended for consumption by a patient.
The following figure diagrams the Substance Administration Order RMIM:
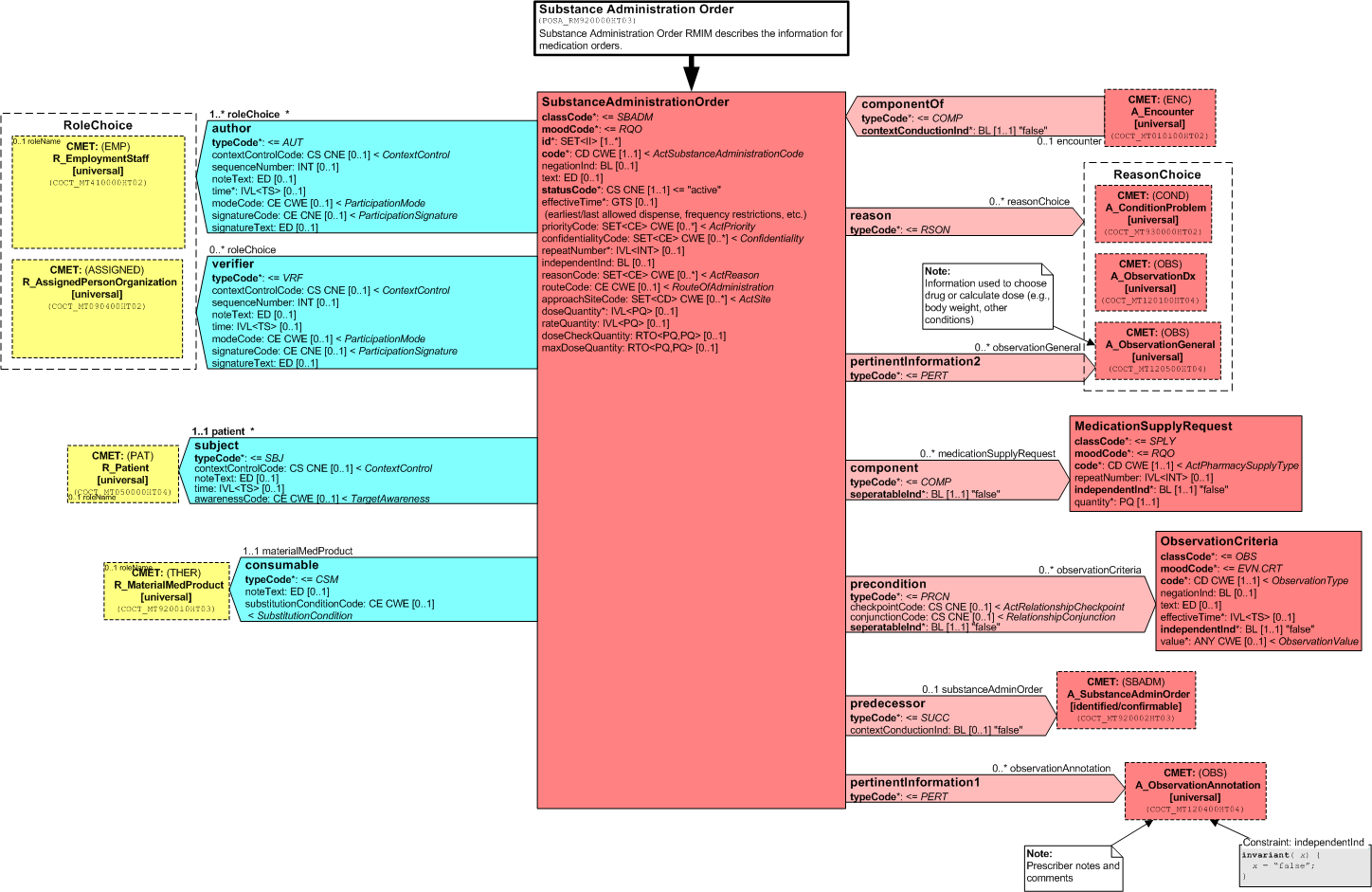
Figure: 4.29.2-1 Substance Administration Order RMIM
The list of trigger events follows:
Table 4.29.3-1 Substance Administration Order - Trigger Events| Begin State | End State | Trigger ID | Trigger Event |
| aborted | nullified | POSA_TE000005 | Nullify Substance Administration Order |
| active | aborted | POSA_TE000003 | Abort Substance Administration Order |
| active | active | POSA_TE000012 | Revise Active Substance Administration Order |
| active | completed | POSA_TE000002 | Complete Substance Administration Order |
| active | nullified | POSA_TE000005 | Nullify Substance Administration Order |
| active | suspended | POSA_TE000004 | Suspend Substance Administration Order |
| completed | active | POSA_TE000013 | Reactivate completed substance administration order |
| completed | completed | POSA_TE000007 | Revise completed substance administration order |
| completed | nullified | POSA_TE000005 | Nullify Substance Administration Order |
| new | active | POSA_TE000008 | Activate new substance administration order |
| null | aborted | POSA_TE000003 | Abort Substance Administration Order |
| null | active | POSA_TE000001 | Activate Substance Administration Order |
| null | completed | POSA_TE000002 | Complete Substance Administration Order |
| null | suspended | POSA_TE000004 | Suspend Substance Administration Order |
| suspended | aborted | POSA_TE000003 | Abort Substance Administration Order |
| suspended | active | POSA_TE000011 | Resume suspended Substance Administration Order |
| suspended | completed | POSA_TE000002 | Complete Substance Administration Order |
| suspended | nullified | POSA_TE000005 | Nullify Substance Administration Order |
| suspended | suspended | POSA_TE000009 | Revise suspended Substance Administration Order |
The list of Interactions follows:
Table 4.29.4-1 Substance Administration Order - Interactions| Interaction ID | Trigger Event | Transmission Wrapper | Control Act Wrapper |
| POSA_IN000001 | POSA_TE000001 | MCCI_MT000100HT04 | MCAI_MT700200HT01 |
| POSA_IN000002 | POSA_TE000002 | MCCI_MT000100HT04 | MCAI_MT700200HT01 |
| POSA_IN000003 | POSA_TE000003 | MCCI_MT000100HT04 | MCAI_MT700200HT01 |
| POSA_IN000004 | POSA_TE000004 | MCCI_MT000100HT04 | MCAI_MT700200HT01 |
| POSA_IN000005 | POSA_TE000005 | MCCI_MT000100HT04 | MCAI_MT700200HT01 |
| POSA_IN000007 | POSA_TE000007 | MCCI_MT000100HT04 | MCAI_MT700200HT01 |
| POSA_IN000008 | POSA_TE000008 | MCCI_MT000100HT04 | MCAI_MT700200HT01 |
| POSA_IN000009 | POSA_TE000009 | MCCI_MT000100HT04 | MCAI_MT700200HT01 |
| POSA_IN000011 | POSA_TE000011 | MCCI_MT000100HT04 | MCAI_MT700200HT01 |
| POSA_IN000012 | POSA_TE000012 | MCCI_MT000100HT04 | MCAI_MT700200HT01 |
| POSA_IN000013 | POSA_TE000013 | MCCI_MT000100HT04 | MCAI_MT700200HT01 |
| Artifacts | Link |
| Visio File | posa_rm920000ht03.vsd |
| Visio XML File | posa_rm920000ht03.xml |
| Hierarchical Message Description (HMD) | posa_hd920000ht03.xls |
| Schema | posa_mt920000ht03.xsd |
| Model Interchange Files (MIF) | posa_mt920000ht03.mif |
| Side Effect Configuration Spreadsheet (for use in WebADI) | posa_mt920000ht03.csv |
| Interaction Schemas | posa_in000001.xsd |
| Contents Previous Next Conformance Examples |
|
Copyright © 2018, Oracle. All rights reserved. |
About Oracle Contact Us Legal Notices and Terms for Use Privacy Statement |