21Recording Product Analysis for Adverse Events and Complaints Management
Recording Product Analysis for Adverse Events and Complaints Management
About Recording Product Analysis for Adverse Events and Complaints Management
This chapter describes how to use the Siebel Business Application during the product analysis phase of an adverse events and complaints investigation.
When a faulty product is returned to the manufacturer for study, a product analysis record is opened. This record documents details of the analysis. When the analysis is complete, root cause and corrective action information are added and the record is submitted for approval.
Scenario for Product Analysis Arising from a Complaint
This scenario is an example process performed by the Siebel administrator, the manager of the product analysis team, a team member, and the quality manager. Your company might follow a different process according to its business requirements.
This process is designed to illustrate the functionality of the Siebel AECM.
Introduction
A customer had a problem with a new blood analyzer system. The analyzer’s cartridge was determined to be faulty, and the customer returned it to the manufacturer for analysis. The quality manager has already opened a product complaint file (product issue record) for this problem.
The Siebel Administrator
The administrator sets up the activity templates that the product analysis team uses in the course of analyzing product issues. Before creating these templates, the administrator consults with the quality manager and reviews the company’s S.O.P (standard operating procedure) for product analysis.
The administrator checks that all the necessary codes for product analysis have been set up as appropriate for the company’s business process and products.
Quality Manager
The initial review of the product complaint has been completed and the faulty cartridge has been sent by the customer. The quality manager creates a product analysis record in the complaint file. She assigns a member of the product analysis team to be the primary owner of the product analysis record.
Member of the Product Analysis Team
The decontamination unit initially receives and processes the faulty cartridge. After decontamination, the cartridge is sent to a member of the product analysis team. He records receipt of the faulty cartridge, reviews the product analysis record associated with this complaint, and begins analysis of cartridge.
During the course of the analysis, the team member creates activities to keep track of the analysis steps. He records the results of the analysis. At the conclusion of the analysis, he records the root cause of the problem and proposes corrective action.
When analysis of the cartridge is complete, the team member submits the product analysis information for approval.
Quality Manager and Product Analysis Manager
Both the manager of the product analysis team and the owner of the product complaint file are required to approve the product analysis. When they review their Inboxes, they learn that the product analysis is complete and ready for approval. Individually, they review and approve the product analysis.
Process of Product Analysis Following a Product Issue
This example process represents the tasks that are carried out in the Scenario for Product Analysis Arising from a Complaint.
Administrator Procedures
Creating Product Analysis Activity Templates
Product analysis activity templates are used to create standard sets of activities that the product analysis team use to guide them as they carry out decontamination procedures, validation tests, and so on.
This task is a step in Process of Product Analysis Following a Product Issue.
To create a product analysis activity template
Navigate to the Administration - Data screen, then the Activity Templates view.
In the Activity Templates list, create a new record and complete the necessary fields.
Set the Type field to Repair.
Leave these fields blank: Sales Stage, Sales Method, and Protocol Title; they do not apply to product analysis activities.
Associate individual activities with the template, as described in Siebel Applications Administration Guide.
Setting Up Codes for Product Analysis
The administrator defines the codes that are used to categorize product analysis.
This task is a step in Process of Product Analysis Following a Product Issue.
To set up codes for Product Analysis
See Setting Up Codes.
Creating Product Analysis Records from a Product Issue
The first task in analyzing a defective product is to create a product analysis record. The product analysis record captures information from the associated product issue and serves as a repository for all information associated with the product analysis.
Typically, the product analysis record is created by the quality manager who has access to the Product Issues screen. The quality manager can then assign the product analysis to a member of the analysis team who has access to the Repairs screen but not necessarily to the Product Issues screen.
The product analysis record is a repair record of type Product Issue Analysis. For information about the Repairs screen, see Siebel Field Service Guide.
This task is a step in Process of Product Analysis Following a Product Issue.
To create and assign the product analysis record
Navigate to Product Issues screen, then the Product Issue List view.
In the Product Issues list, drill down on a product issue.
Click the Product Analysis tab.
In the Repairs list, create a new product analysis record and complete the necessary fields.
Account, Product, Asset number, Lot number, and Service Request number are copied from the product issue to the new product analysis record.
In the Repairs list, drill down on the product analysis record (using the Repair # field link).
Complete the Owners field.
If Assignment Manager is set up, then you can assign an owner by clicking the cogwheel icon, and selecting Assign.
Filling in Product Analysis Records
The product analysis team member records information such as the results of decontamination and validation tests.
This task is a step in Process of Product Analysis Following a Product Issue.
To complete a product analysis
Navigate to Repairs screen, then the Repair List view.
In the Repair list, select a product analysis record.
Complete the fields.
Some fields are described in the following table. Other fields are described in Siebel Field Service Guide.
Field Comments Codes
Codes to describe or categorize the product analysis. Typically, select codes of type Product Analysis.
Product
The primary product associated with the product issue.
Received
Defaults to the date and time that the record was created.
Repair #
A unique identifying number for the repair. Automatically populated.
Type
Defaults to Product Issue Analysis.
Drill down on the Repair # field to open the record.
Select the Activity Plans tab and create activity records as required.
Select the Attachments tab and attach documents as required.
Select the Notes tab and add notes as required.
Completing Product Analysis and Creating Correctiveand Preventive Actions
At the conclusion of the product analysis, the product team member adds information about the root cause of the problem and initiates corrective and preventive actions (CAPA).
This task is a step in Process of Product Analysis Following a Product Issue.
To add root cause and corrective actions to a product analysis record
Navigate to Repairs screen, then the Repair List view.
In the Repair list, drill down on a product analysis record and complete the Root Cause field.
Add corrective actions to the record as follows:
Click the Corrective Actions tab.
In the Corrective Actions list, add an existing or create a new corrective action as required.
If the corrective action... Then... And... Already exists
Add an existing corrective action
Select a corrective action from the Add Corrective Actions window
Is new
Create a new corrective action
Complete the fields in the new CAPA record
For more information about CAPAs, see Managing Corrective and Preventative Actions
Submitting Product Analysis Records
Once the root cause has been recorded and corrective actions initiated, the product analysis is ready to be submitted for approval.
Here are some restrictions on submitting a product analysis record:
Only the primary owner of the product analysis record can submit it.
The owner of the product analysis must have a manager.
The product analysis must have a product issue associated with it.
The associated product analysis must have an owner.
This task is a step in Process of Product Analysis Following a Product Issue.
To submit a product analysis
Navigate to Repairs screen, then the Repair List view.
In the Repair list, select a product analysis.
Click Submit.
This starts a workflow (LS Medical PA Submit) that sends requests for approval to the Inboxes of the designated approvers. For more information about the workflow, see About Configuring Product Analysis Approvals.
Note: If you submit a product analysis record in error, click the Withdraw button to unsubmit it. This starts the LS Medical PA Withdraw workflow. For more information about the workflow, see About Configuring Product Analysis Approvals.Verify that the product analysis was submitted to the correct approvers:
Drill down on the product analysis record.
Click the Approval History tab.
The list should contain three records for:
The primary owner (This should already be approved)
The primary owner's manager
The product issue's primary owner
Approving or Rejecting Product Analysis Records
Depending on your business process, an approved product analysis might be required before corrective actions can be taken.
In the preconfigured application, the required approvers for product analysis records are:
The manager of the product analysis primary owner
The primary owner of the product issue record that generated product analysis
This task is a step in Process of Product Analysis Following a Product Issue.
Approving Product Analysis Records
The following procedure shows you how to approve a product analysis record.
To approve a product analysis record
Navigate to Inbox screen, then the Inbox Items List view.
In the Inbox Items List, review and approve the item.
The status of the product analysis becomes Closed and the sub status becomes Approved.
Rejecting Product Analysis Records
The following procedure shows you how to reject a product analysis record.
To reject a product analysis record
Navigate to Inbox screen, then the Inbox Items List view.
In the Inbox Items List, review and reject the item.
The status of the product analysis changes to Rejected. In such cases, the product analysis team member must submit the record again.
If Manager 1 rejects the item before Manager 2 takes action on it, Manager 2 never sees the item (it is deleted from Manager 2’s Inbox when it is rejected by Manager 1).
For more information about the Inbox, see Siebel Applications Administration Guide.
About Configuring Product Analysis Approvals
There are two buttons associated with adverse events and complaints investigation. You can configure the workflows for these buttons.
Submit button (LS Medical PA Submit Workflow)
Withdraw button (LS Medical PA Withdraw Workflow)
You can modify workflows to suit your own business model using Siebel Business Process Designer. For more information, see Siebel Business Process Framework: Workflow Guide.
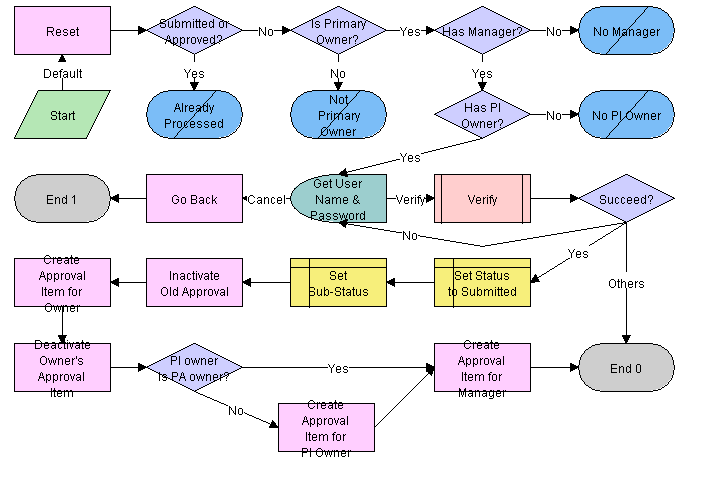
LS Medical PA Submit Workflow
This workflow is initiated from the Submit button on the Repairs screen.
The workflow appears in the following figure.
Workflow Description
This workflow performs the following actions:
Checks if the current record has already been submitted. If it has, the workflow ends.
Checks if the current record has an owner, if the owner has a manager, and if the product issue record that generated this record has a primary owner. If these criteria are not met, the workflow ends.
Calls the LS Medical User Verification workflow. If the authentication does not pass, the workflow ends.
Sets the status to Submitted and the sub-status to Waiting.
Deactivates any approval record associated with the current product analysis record.
Creates an inactive approval record for the current owner (for record purposes).
Creates approval items for the owner’s manager and for the owner of the product issue.
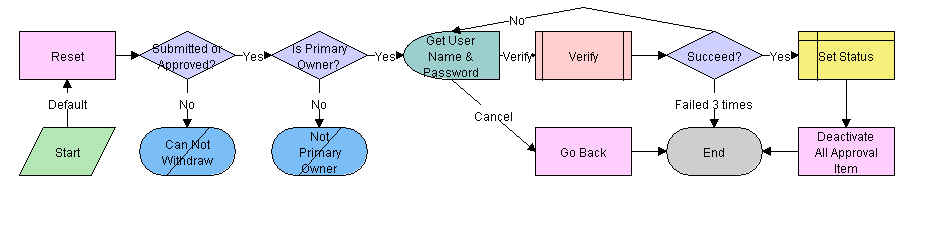
LS Medical PA Withdraw Workflow
This workflow is initiated from the Withdraw button on the Repairs screen.
The workflow appears in the following figure.
Workflow Description
This workflow performs the following actions:
Checks if the current record has already been submitted. If it has not, the workflow ends.
Checks if the user is the primary owner. If not, the workflow ends.
Calls the LS Medical User Verification workflow. If the authentication does not pass, the workflow ends.
Sets the status to Reopen.
Deactivates any approval record associated with the current product analysis record.