19Capturing Adverse Events and Complaints
Capturing Adverse Events and Complaints
This chapter covers the following topics:
About Adverse Events and Complaints Management
Adverse events and complaints management (AECM) is an important process in the regulated medical device and pharmaceutical industries. The FDA in the USA and similar agencies in other countries have specific requirements for capturing, investigating, reporting, and resolving adverse events and complaints on medical devices and drugs.
Poor handling of adverse events and complaints can lead to non-compliance penalties such as warning letters, restrictions, fines, and recalls.
Siebel Adverse Events and Complaints Management
Siebel Adverse Events and Complaints Management (Siebel AECM) is designed to manage the life cycle of an adverse event or a medical product complaint. Users collaborate and capture all of the necessary data to track the complaint through investigation to corrective action and generate the required regulatory reports. The following illustrates such a life cycle. Each step in the process represents a chapter in this book.
Table Complaints Process Flow
| Process | Roles | Typical Activities |
|---|---|---|
1. Capture adverse event or complaint |
Call Center Agent |
|
2. Investigate adverse event or complaint |
Quality or Regulatory Agency |
|
3. Manage product analysis and CAPA |
Product Analysis and CAPA Team |
|
4. Report to regulatory agency |
Quality or Regulatory Agency |
|
5. Manage customer communication |
Call Center Agent or Quality or Regulatory Agency |
|
6. Close adverse event or complaint |
Quality or Regulatory Agency |
|
Integration with Other Siebel Business Applications
Siebel AECM is integrated with Siebel Call Center, Siebel Sales, Siebel Field Service, and Siebel Clinical. This allows the flexibility to share varying levels of visibility to adverse event or complaint details, investigation status, and corrective action across different users and functions within the organization. The Siebel Audit Trail functionality keeps a record of changes made to records throughout the course of the investigation.
About Capturing Adverse Events and Complaints
This chapter describes capturing information about a medical product complaint or adverse event as a service request and then escalating that complaint as a product issue for further investigation.
One of the goals in capturing such information is to identify complaints accurately, making sure that all valid complaints are identified while at the same time minimizing the number of false-positive complaints that get escalated for investigation.
Siebel AECM Terms
The following information lists some important terms and abbreviations used in Siebel AECM.
Table Siebel AECM Terms
| Term | Description |
|---|---|
Adverse Event |
An adverse event is a customer event associated with a product malfunction or adverse reaction to a pharmaceutical. It can be initially captured as a service request and then escalated to a product issue (which becomes the complaint file associated with the event). In some places in this text, complaints includes both adverse events and complaints. |
AECM |
Adverse Events and Complaints Management |
Call Center Agent |
Another name for this job title is customer service agent. This agent is trained to identify a product complaint and distinguish it from a normal service or information request. |
CAPA |
Corrective and preventive action. This specifies the changes in product or process needed to resolve the root cause of the adverse event or complaint. For example, a CAPA could be a request from the analysis team for the correction of the manufacturing, design, or process flaw that caused the initial adverse event or complaint. |
Complaint |
A complaint is a customer event associated with a product malfunction. It can be initially captured as a service request and then escalated to a product issue (which becomes the complaint file associated with the event). In some places in this text, complaints includes both adverse events and complaints. |
Initial regulatory report |
The first report to a regulatory agency regarding a product complaint or an adverse event. |
MDR |
Medical device reports are required to be submitted to a regulatory agency when an adverse event or complaint is determined to be reportable. |
MDV |
Medical Device Vigilance |
Medical Product |
Examples of medical products are capital equipment, disposables, reagents, implanted devices, and pharmaceuticals. |
MedWatch 3500A |
U.S. Food and Drug Administration (FDA) report for mandatory reporting of adverse reactions and medical product problems by drug and biologic manufacturers and packers and by medical device manufacturers, distributors, importers, and user-facilities. |
MedWatch 3500A Supplemental |
A 3500A report sent as a follow-up to an initial 3500A report. |
NCA |
National Competent Authorities. Regulatory reports are often sent to these agencies. These country-specific agencies also report adverse events to each other. |
Product Issue |
A product issue is the adverse event or complaint file. The product issue is often first captured as a service request record. Then the information is transferred to a product issue record, which becomes the adverse event or complaint file. |
Product Issue Assessments |
A product issue assessment evaluates a product issue on defined criteria. For example, a product issue assessment can be used to determine whether the product issue should be reported to a regulatory agency. |
Quality Manager |
Other names for this job title are complaint investigator, regulatory affairs representative, safety and regulator manager, regulatory liaison, and quality group liaison. |
Regulatory report |
There are four types of regulatory reports supported by Siebel AECM (preconfigured):
Regulatory report record. The record in the Regulatory reports screen. Regulatory report. The report created using data contained in the regulatory report record. Other regulatory reports like CIOMS can be configured using other reporting tools. |
Siebel AECM |
The Siebel Adverse Events and Complaints Management module. |
Signature capture |
See User authentication. |
SmartScripts |
A SmartScript is a collection of predefined questions, answers, and branches that can be used to guide the quality manager through potentially complex decision making processes to arrive at a solution to a problem or query. For more information about SmartScripts, see Siebel SmartScript Administration Guide. |
Inbox |
The Inbox allows managers and other employees to view and approve items (of multiple types) from one screen. In Siebel AECM, the Inbox is used to approve product analyses. For more information, see Siebel Applications Administration Guide. |
User authentication |
The user authorization check captures and verifies the user's name and password. This user authentication is controlled by the LS Medical User Verification workflow.
Note: User authentication is sometimes called signature capture, but should not be confused with electronic signature capture on mobile devices or tablet PCs used elsewhere in the
Siebel Life Sciences application.
|
Scenario for Capturing and Escalating Adverse Events and Complaints
This scenario is an example workflow performed by the Siebel administrator and a call center agent. Your company might follow a different workflow according to its business requirements.
This workflow is designed to illustrate the functionality of Siebel AECM.
Introduction
A medical manufacturing company makes capital equipment for use in hospitals. The company has recently released a new blood analyzer system. The company manufactures both the instrument and the consumables.
The Siebel Administrator
In preparation for the new blood analyzer being released on the market, the Siebel administrator performs a number of preparatory administration tasks so that the product and all necessary codes and lot numbers are set up in the Siebel Business Application. In case the company has to file a 3500A report, the administrator refers to the MedWatch coding manual and sets up appropriate codes for event problems and evaluation.
The Siebel administrator also sets up the activity templates used by the call center agents to create activity plans for handling customer complaints. The Siebel administrator needs to have all these ready before the call center agents begin to receive calls about the product.
When troubleshooting and problem resolution information becomes available for the new blood analyzer, the Siebel administrator creates solution records that contain this troubleshooting information.
The Call Center Agent
A call center agent takes a customer call about a failing blood analyzer system.
In addressing the customer’s complaint, the call center agent does the following:
Enters a service request, recording details about the product and the failure.
Works with the customer to troubleshoot the problem, searching the solution database for possible solutions. In this case, he finds a solution document about running a diagnostic test. The test does not resolve the problem, but it does indicate the repair that is needed.
Creates an activity plan—a list of predefined activities—to dispatch a service engineer to the customer site.
Dispatches a service engineer to make a repair to the analyzer.
Sets up an RMA (return materials authorization) to return the cartridge and reagents that were in use when the analyzer failed.
Reviews the report of the work done by the field engineer.
The call center agent has been trained to identify calls as potential complaints. He recognizes that this customer’s problem needs to be escalated as a product issue. Before escalating, he reviews the information entered in the service request, making sure that the service request is classified correctly, for example, with accurate types and codes. When the call center agent creates the product issue from the service request record, the appropriate data is transferred from the service request to the product issue record.
Process of Capturing and Escalating Adverse Events and Complaints
This example process represents the tasks that are carried out in the Scenario for Capturing and Escalating Adverse Events and Complaints.
Administrator Procedures
The Siebel administrator sets up the service request functionality so that call center agents can capture adverse events and complaints. To do this, the Siebel administrator performs the following tasks:
End-User Procedures
To capture and escalate adverse events and complaints, end users perform the following tasks:
Confirming Standard Setup for Service Requests
This task is a step in Process of Capturing and Escalating Adverse Events and Complaints.
Before service requests can be logged against products, the Siebel administrator sets up the following:
Products. Records need to be set up for products against which there might be adverse events and complaints.
Product Lines and Categories. Product lines and categories can both be associated with codes. If you configure your application to filter codes according to product line or category, make sure to set these up. For more about codes, see Setting Up Codes.
Assets. Asset records should be set up for devices with serial numbers. Assets do not need to be set up for items such as consumables.
Activity Templates of type Service Request. Call center agents use activity templates to create service request activity plans—sets of predefined and scheduled activities associated with service requests.
Solutions. Many service requests are similar to previously addressed issues. Solution documents contain information about previously resolved requests.
LOVs for Type, Area, and Sub Area. These fields are particularly important for classifying service requests. You can customize these LOVs.
You might have already completed these setup tasks for other reasons in Siebel Life Sciences. These setup tasks are standard in many Siebel Business Applications and are not unique to Siebel Life Sciences.
Setting up Products, Product Lines, Categories, Assets, Activity Templates, Solutions, and LOVs
For information, consult the following table.
Table Setting up Products, Product Lines, Categories, Assets, Activity Templates, Solutions, and LOVs
| To Set Up... | Use This Screen... | And for More Information, See... |
|---|---|---|
Products |
Product Administration |
Defining External Products and Siebel Product Administration Guide |
Product Lines |
Product Administration screen, then Product Lines |
Siebel Product Administration Guide |
Categories |
Catalog Administration |
|
Assets |
Assets |
Siebel Field Service Guide, Siebel Communications Guide |
Activity Plans of type Service Request |
Data Administration screen, then Activity Templates |
Siebel Field Service Guide |
Solutions |
Solution Administration |
Siebel Field Service Guide |
LOVs |
Data Administration screen, then List of Values |
Siebel Applications Administration Guide |
Setting Up Codes
This task is a step in Process of Capturing and Escalating Adverse Events and Complaints.
Codes allow you to codify many aspects of the AECM process. For example, codes are used to categorize the service request that triggers the adverse event or complaint, and codes are used to categorize the corrective actions initiated at the end of a complaint’s life cycle.
Multiple codes and sometimes codes of multiple types can be specified in one code field.
Think carefully when designing and setting up your code system. Codes are used as search criteria for service requests and product issues.
According to your business needs, you can use:
Industry codes such as FDA patient, device, method, results, and conclusion codes required for the MedWatch 3500A form
Company-specific codes
A combination of industry and company-specific codes
The following table lists where code fields appear in the Siebel AECM module.
Table Code Fields in the Siebel AECM Module
| Screen | View | Field Name | Notes |
|---|---|---|---|
Service Requests |
More Info |
Codes |
None. |
Product Issues |
More Info |
Codes |
Copied from the service request when a product issue is created. Codes from the service request record are copied to this field when a product issue is created from a service request. |
Product Issues |
Corrective Actions |
Codes |
Typically, codes of type Corrective Action are entered in this field, although any type of code can be entered. |
Product Issues |
Investigation |
Method Codes |
Only codes of type Method can be entered. |
Product Issues |
Investigation |
Result Codes |
Only codes of type Result can be entered. |
Product Issues |
Investigation |
Conclusion Codes |
Only codes of type Conclusion can be entered. |
Product Issues |
Investigation |
Non-Evaluation Codes |
Only codes of type Non-Evaluation can be entered. |
Product Issues |
Importer MDV |
Patient Codes |
Only codes of type Patient can be entered. |
Product Issues |
Device Codes |
Only codes of type Device can be entered. |
|
Repairs |
More Info |
Codes |
Typically, codes of type Product Analysis are entered in this field, although any type of code can be entered. |
Corrective Actions |
More Info |
Codes |
Typically, codes of type Corrective Action are entered in this field, although any type of code can be entered. |
Regulatory Reports |
More Info |
Method Codes |
Only codes of type Method can be entered. Copied from the product issue when a regulatory report is populated. |
Regulatory Reports |
More Info |
Result Codes |
Only codes of type Result can be entered. Copied from the product issue when a regulatory report is populated. |
Regulatory Reports |
More Info |
Conclusion Codes |
Only codes of type Conclusion can be entered. Copied from the product issue when a regulatory report is populated. |
Regulatory Reports |
More Info |
Non-Evaluation Codes |
Only codes of type Non-Evaluation can be entered. Copied from the product issue when a regulatory report is populated. |
Regulatory Reports |
Importer MDV |
Patient Codes |
Only codes of type Patient can be entered. Copied from the product issue when a regulatory report is populated. |
Regulatory Reports |
Importer MDV |
Device Codes |
Only codes of type Device can be entered. Copied from the product issue when a regulatory report is populated. |
Activities |
Items |
Code |
Single value field. |
An Example
When you set up codes, you can enter values for Product, Product Line, Category, Type, Area, and Sub Area that are associated with the code. Call center agents can use these values to search for and identify the correct code for a service request.
The following table is an example of the field values used to describe one code.
Table Example Fields for a Code Classifying a Pump Error on a Blood Analyzer
| Field | Example Value |
|---|---|
Name |
Error 333 |
Description |
Blood analyzer pump overflow. |
Code Type |
Device |
Product |
Blood Analyzer Model 350, Blood Analyzer Model 373. This field can have multiple values. |
Product Line |
Blood analyzer, Dialysis system This field can have multiple values. |
Category |
Nephrology |
Type |
Potential Complaint |
Area |
Software |
Sub Area |
Pump timing |
How to Set Up Codes
Create the codes needed to classify your company’s adverse events and complaints.
Codes are needed for the procedures in this chapter and for procedures in the following AECM chapters.
To set up codes
Navigate to Administration - Service screen, then the Code Administration view.
In the Code Admin list, create a new record and complete the necessary fields.
Some fields are described in the following table.
Field Comments Name
Enter the name for the code. Typically this is a number or a letter number combination.
Code Type
Examples are: Method, Result, Conclusion, Device, Patient.
Product
Multiple products can be associated with a code.
Product Line
Multiple product lines can be associated with a code.
Category
A grouping of products. Only one category can be associated to a code. To set up categories, see Siebel Order Management Guide.
Type
Use to categorize the type of service request, for example, as a potential complaint. This is the same LOV used in service request and product issue records.
Area
The Type field constrains this drop-down list.
Sub Area
The Area field constrains this drop-down list.
Setting Up Lot Numbers for Medical Products
Create lot numbers for all batches of products that are tracked by lot number.
Lot numbers for Siebel Medical and Siebel Pharma are the same except that:
| For... | Lot numbers are set up in ... |
|---|---|
Siebel Medical |
Administration - Service screen, then the Lot Administration view |
Siebel Pharma |
Administration - Samples screen, then the Lot Setup view |
This task is a step in Process of Capturing and Escalating Adverse Events and Complaints.
To set up lot numbers
Navigate to Administration - Service screen, then the Lot Administration view.
In the Lot Admin list, create a new record and complete the necessary fields.
Some fields are described in the following table.
Field Comments Expiration Date
This information can be used for recalls of expired products.
Effective Start Date
The date of manufacture, the date on which the product was received, or another date defined by your company.
Lot #
Enter an unique value that corresponds to the lot number printed on the label of the product.
Product
The name of the product with which this lot number is affiliated.
For more information, see Defining Internal Products paying specific attention to Product Categorization Settings and About Samples and Promotional Items Settings.
Product Level
Applies only to drug samples.
Capturing Adverse Events and Complaints as Service Requests
The creation of the service request by the call center agent is often the first step in AECM.
The following procedure assumes that you are familiar with the service request screen and associated functionality. (For information about the service request screen, see Siebel Field Service Guide.)
This task is a step in Process of Capturing and Escalating Adverse Events and Complaints.
To create a service request
Navigate to the Service Request screen, then the Service Request List view.
In the Service Request list, create a new record and complete the necessary fields, such as account, contact, product, summary, and description.
Some fields are described in the following table.
Field Comments Codes
Codes to describe or categorize the service request.
Lot #
Select an existing lot number or enter a new lot number.
You can enter a new lot number in one of two ways:
Formally enter a new lot number using the New button on the Lot Setup dialog box.
Use the Lot # field as a simple text field. If you enter a Lot # this way, the value is only stored in this product issue record and is not checked against a table of valid lot numbers. This does not create a new lot # record in the Lot Administration view.
Product Issue
This is automatically flagged if the service request is escalated to a product issue.
Type
Use this field to categorize the type of service request, for example, as a potential complaint.
Area
The Type field constrains this drop-down list.
Sub Area
The Area field constrains this drop-down list.
Search for a solution to the service request:
Drill down on a record.
Click the Solutions tab.
In the Solutions list, create a new record and complete the necessary fields.
In the Add Solutions dialog box that appears, query for a solution and add it.
In the Solutions list, drill down on the Name field to see details about the solution.
For more information about solutions, see Siebel Field Service Guide.
Set up activities for the service request using an activity plan.
For example, appointments for the field service engineer can be set up from the Activities view. For more information about field service activities, see Siebel Field Service Guide.
Dispatch a field engineer.
For more information about dispatch and scheduling, see Siebel Field Service Guide.
If products or materials need to be returned for analysis, set up an RMA order.
For more information about shipping and receiving, see Siebel Field Service Guide.
Escalating Adverse Events and Complaints as Product Issues
A valid adverse event or complaint that is first recorded as a service request should be transferred to a product issue record. As a product issue, it can be investigated by the quality manager. This allows the Call Center to retain ownership of the service request and the Quality team to manage the investigation and processing of the complaint.
This procedure describes creating a product issue from the Service Requests screen. Product issue records can also be created in the Product Issues screen and the Site Management screen.
This task is a step in Process of Capturing and Escalating Adverse Events and Complaints.
To create a product issue from a service request
Navigate to the Service Requests screen, then the Service Request List view.
In the Service Request list, select the record for which you want to create a product issue.
Click Create Product Issue.
This starts the Create a Product Issue from a Service Request workflow that:
Makes these fields in the Service Request read-only:
Account
Last Name
First Name
Product
Creates a product issue record from a Service Request.
Copies these fields from the service request to the new product issue:
Account
Type
Product
Summary
Area
Codes
Last Name
Sub Area
Asset #
First Name
Priority
Lot #
Description
Severity
Takes you to the Product Issues screen.
For more information about the workflow, see About Configuring Adverse Events and Complaints Capture.
Complete the necessary fields in the product issue record.
Typically, call center agents enter data such as the event date, the outcome, and whether the product was returned. If more than one product is involved with the adverse event or complaint, it is entered in the product issue record. (Only one product can be associated with a service request record.)
Adding Complaint-Specific Information to Product Issues
The call center agent enters complaint-specific information to the product issue record, for example, patient details. Information about only one patient can be associated with a product issue.
This task is a step in Process of Capturing and Escalating Adverse Events and Complaints.
To add information about the patient to the Product Issue record
Navigate to Product Issues screen, then the Product Issue List view.
In the Product Issues list, drill down on a product issue.
Note: If you created the product issue using the Create Product Issue button in the Service Requests screen, the workflow takes you to this view.Click the Patient tab.
Complete the fields in the Patient form.
Some fields are described in the following table.
Field Comments Mapping to 3500A Form Patient Identifier
A1
Gender
A3
Age
A2
Date of Birth
A2
Weight
A4
U/M
Unit of measurement for weight
A4
About Configuring Adverse Events and Complaints Capture
There are two aspects of the Create Product Issue button that you can configure:
The Create a Product Issue from a Service Request Workflow
The fields that are copied from the Service Request to the Product Issue record
Create a Product Issue from a Service Request Workflow
This workflow (LS Medical Create PI from SR) is initiated from the Create Product Issue button on the Service Requests screen.
The workflow appears in the following figure.
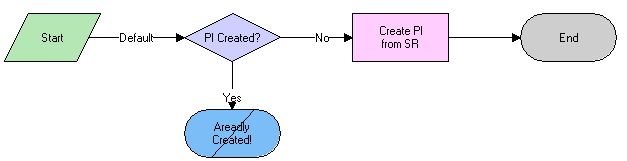
Workflow Description
This workflow performs the following actions:
Checks if a product issue has already been created from this service request. If it has, the workflow ends. (Only one product issue can be created from a service request using the Create Product Issue button.)
Creates a new product issue.
Copies data from the service request to the product issue.
Goes to the Product Issue screen, then the More Info view.
You can modify workflows to suit your own business model using Siebel Business Process Designer. For more information, see Siebel Business Process Framework: Workflow Guide.
Changing Which Fields Are Copied to the Product Issue
When the Create Product Issue button is used to create a product issue record from a service request record, data from various fields are copied from the service request record to the new product issue record.
To change which fields are copied when a product issue is created
Use the Data Transfer Utilities to edit the LS Medical Create PI from SR data map object.
For information about the Data Transfer Utilities, see Siebel Finance Guide.